MATERIALS AND METHODS
advertisement

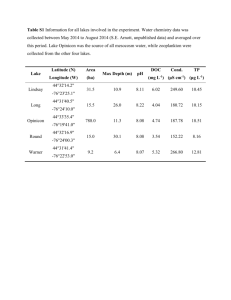
EXPERIMENTAL PROCEDURES Data sources and phylogenetic analyses Genome sequences for the bryophytes P. patens, the lycophyte S. moellendorffii and the selected microalgae were retrieved from the websites listed in Table S1. A local blast database was constructed. Proteins from all genomes were blasted with the Arabidopsis proteins (Table S2). Blast results were parsed for ≥25% amino acid identity with E-values ≤1e-10. The CDD (Conserved Domain Database; http://www.ncbi.nlm.nih.gov/cdd/), Pfam (http://pfam.sanger.ac.uk/search) and SMART (http://smart.emblheidelberg.de/) databases were used to search for structurally conserved domains within specific proteins. Sequences ultimately selected are listed in Data S1. Parsed hits for all species were aligned using CLUSTALW (Chenna et al. 2003). Gaps and ambiguously aligned sites were removed using gBlock (Talavera and Castresana 2007). Sequences that caused aberrant alignments and whose real identity could not be confirmed were removed manually. ProtTest (Darriba et al. 2011) was used to select models of protein substitution and rate heterogeneity that best fit each data set. Phylogenetic analyses were performed with a maximum likelihood method using PhyML 3.0 (Guindon et al. 2010). Bootstrap support values were estimated using 100 pseudo-replicates. The number of paralogs in each case was determined by counting the number of sequences for each lineage in the ortholog clade. Abscisic acid (ABA) analysis Samples were analyzed for ABA using a modified version of the method described by Turečková et al (Turečková et al. 2009). Briefly, samples were extracted in 1 mL cold methanol/water/acetic acid (10/89/1, v/v/v) and 20 pmol of [2H6] (+) ABA was added as an internal standard. After 1 h, the homogenates were centrifuged and the pellets were re-extracted for another hour. The combined supernatants were then cleaned on Oasis® HLB cartridges (60 mg, 3 ml, Waters, Milford, MA, USA). They were then methylated, purified by ABA-specific immunoaffinity extraction (Hradecká et al. 2007) and analysed by UHPLC-ESI (+)-MS/MS (Micromass, Manchester, UK). Cytokinin (CK) analysis Samples were analyzed for their CK content as described by Novák et al (Novák et al. 2008). In brief, 500 mg (DW) was extracted overnight in Bieleski’s extraction solution. Stable isotope-labeled CK internal standards (IS) were added (3 pmol of each compound per sample in the case of the CK bases, ribosides and O-glucosides, and 5 pmol of each CK nucleotide). The following IS were used: [13C5]tZ, [2H5]tZR, [2H5]tZ9G, [2H5]tZOG, [2H5]tZROG, [2H5]tZRMP, [13C5]cZ, [2H3]DHZ, [2H3]DHZR, [2H3]DHZ9G, [2H7]DHZOG, [2H3]DHZRMP, [2H6]iP, [2H6]iPR, [2H6]iP9G, [2H6]iPRMP, [2H7]BA, [2H7]BAR, [2H7]BA9G, [2H7]BARMP, [15N4]mT and [15N4]oT. The supernatant was purified using SCX cartridges (1g per 6ml Bond Elut, Agilent Technologies, CA, USA) and a weak anion-exchange column (DEAE A-25, GE Healthcare, Sweden) on C18 (200mg per 6ml Bond Elut Plexa, Agilent Technologies, CA, USA). The nucleotide fraction was then enzymatically treated (alkaline phosphatase from E. coli - 10U per 5ml of sample) and purified on Plexa C18 (200mg per 6ml). Finally, immunoaffinity chromatography using monoclonal antibodies raised in a mouse was performed to obtain pure extracts for UHPLC-(+)ESI-MS/MS analysis of CKs. Dynamics of phytohormone metabolism transcripts under nitrogen-deleted and nitrogen-replete conditions via mRNA-Seq based time-series transcriptomes Members of Nannochloropsis sp. are heterokonts and are found widely in the marine environment as well as in fresh and brackish waters. These algae are of industrial interest due to their ability to grow rapidly, synthesize large amounts of TAG and high-value polyunsaturated FA (e.g. eicosapentaenoic acid; EPA), and tolerate broad environmental and culture conditions (Radakovits et al. 2012, Vieler et al. 2012, Wang et al. 2012, Wang et al. 2014). Nannochloropsis oceanica IMET1, an industrial strain of EPA and lipid production (Wang, et al. 2014), was selected as a model here for probing hormone metabolism in microalgae. It was inoculated into modified f/2 liquid medium, which was prepared with 35 g l-1 sea salt, 1 g l-1 NaNO3, 67 mg l-1 NaH2PO4*H2O, 3.65 mg l-1 FeCl3*6H2O, 4.37 mg l-1 Na2EDTA*2H2O, trace metal mix (0.0196 mg l-1 CuSO4*5H2O, 0.0126 mg l-1 NaMoO4*2H2O, 0.044 mg l-1 ZnSO4*7H2O, 0.01mg l-1 CoCl2, and 0.36 mg l-1 MnCl2*4H2O), and vitamin mix (2.5 µg l-1 VB12, 2.5 µg l-1 biotin, and 0.5 µg l-1 thiamine HCl). The cells were grown in liquid cultures under continuous light (approximately 50 µmol photons m-2 s-1) at 25˚C and aerated by bubbling with a mixture of 1.5% CO2 in air. Mid-logarithmic phase algal cells were collected and washed three times with axenic seawater. Equal numbers of cells were re-inoculated in nitrogen replete medium (N-replete condition, or N+) and nitrogen-deprived medium (N-depleted condition, or N-) with 50 µmol m-2 s-1 light intensity. Cell aliquots were collected for RNA isolation after being transferred to the designated conditions for 3, 4, 6, 12, 24, and 48 h. Three biological replicates of algal cultures were established under each of the above N+ and N- conditions, respectively. Total algal RNA under the above conditions was extracted using Trizol reagents (Invitrogen). For mRNA-Seq, the poly (A)-containing mRNA molecules were purified using Sera-mag Magnetic Oligo (dT) Beads (Thermo Scientific) and were fragmented into 200- to 300-bp fragments by incubation in RNA Fragmentation Reagent (Ambion) according to the manufacturer’s instructions. The fragmented mRNA was then purified away from the fragmentation buffer using Agencourt® RNAClean beads (Beckman Coulter). The purified, fragmented mRNA was converted into double-stranded cDNA using the SuperScript Double-Stranded cDNA Synthesis Kit (Invitrogen) by priming with random hexamers. Strand nonspecific transcriptome libraries were prepared using the NEBNext® mRNA Library Prep Reagent Set (New England Biolabs) and sequenced for 2×90bp runs (paired-end, PE) using Illumina HiSeq2000. Additional details on the methods and analysis of this time-series transcriptome dataset were provided in (Li et al. 2014), which was published during the review of this submission. Physiological responses of N. oceanica to exogenous ABA and CKs Cells were synchronized by alternating light/dark (12 h/12h) cycles with a final extended dark period (Umen and Goodenough 2001, Zachleder 1994). The following three experiments were performed separately: (i) Synchronous cells (with an initial concentration of 1×106 ml-1) were cultured in flask supplemented with 6-benzyladenine (BA, an artificial CK), ABA or an equivalent amount of HCl or DMSO under 50 μmol photons m-2s-1 light. Cell aliquots were collected for DW determination after five days or for cell cycle analysis by flow cytometry as described at indicated time (Marie et al. 2001). Cell division in randomly selected fields was investigated microscopically at 100× magnification. (ii) Synchronous cells were inoculated in fresh medium and cultured to a concentration of 5×107 ml-1, and then were collected and cultured in photobioreactors suspended in N+ or N- medium respectively. BA was applied to cells under N-. Cell aliquots were diluted and cell numbers were determined under a microscope using a counting chamber. DW were determined after 144 h. (iii) Cells at 5×107 ml-1 were harvested and re-suspended into fresh N+ medium containing 0.5 μM or 5 μM ABA under high light (150 or 300 µmol photons m-2 s-1) or into N- medium containing 0.5 μM or 5 μM ABA under darkness or 50 µmol photons m-2 s-1 light. The Fv/Fm values were determined using Imaging PAM. Analysis of DNA content and cell cycle of N. oceanica by flow cytometry The DNA content and cell cycle were determined using flow cytometry (FACSAria, BD Biosciences) as described by (Marie et al. 2001) with some modifications. (i) Cell aliquots were collected and washed with PBS solution (pH 7.4~7.6) once. (ii) Samples were fixed by adding 2.5% glutaraldehyde (v/v), frozen in liquid nitrogen and stored at −80°C for delayed analysis. Frozen samples were thawed at 37°C. (iii) 10 μl RNase A (10 mg/mL, DNase and protease-free, Thermo Scientific) was added for every 1 ml of sample. Incubation was at 37°C for 30 min. (iv) 10 μl SYBR Green I working solution and 30 μl 1 M potassium citrate was added and the reactions incubated for 15 min at room temperature in the dark. (v) 10 μl of a 1 × 105 beads/ml suspension of 0.95-μm fluorescent microspheres per 1 ml of sample was added. (vi) The sample was analyzed for 4 min at a low running rate (<50 μl/min), and 10,000 cells were analyzed for each sample. Data were collected and cell phase analyzed with MODFIT. REFERENCES Chenna, R., et al. (2003) Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res, 31, 3497-3500. Darriba, D., et al. (2011) ProtTest 3: fast selection of best-fit models of protein evolution. Bioinformatics, 27, 1164-1165. Guenin, S., et al. (2009) Normalization of qRT-PCR data: the necessity of adopting a systematic, experimental conditions-specific, validation of references. J Exp Bot, 60, 487-493. Guindon, S., et al. (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol, 59, 307-321. Hradecká, V., et al. (2007) Immunoaffinity chromatography of abscisic acid combined with electrospray liquid chromatography–mass spectrometry. J Chromatogr B, 847, 162-173. Li, J., et al. (2014) Choreography of transcriptomes and lipidomes of Nannochloropsis reveals the mechanisms of oleaginousness in microalgae. Plant Cell, doi:http://dx.doi.org/10.1105/tpc.113.121418. Marie, D., et al. (2001) DNA/RNA analysis of phytoplankton by flow cytometry. In Current Protocols in Cytometry: John Wiley & Sons, Inc. Novák, O., et al. (2008) Cytokinin profiling in plant tissues using ultra-performance liquid chromatography–electrospray tandem mass spectrometry. Phytochemistry, 69, 2214-2224. Radakovits, R., et al. (2012) Draft genome sequence and genetic transformation of the oleaginous alga Nannochloropis gaditana. Nat Commun, 3, 686. Talavera, G. and Castresana, J. (2007) Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst Biol, 56, 564-577. Trapnell, C., et al. (2009) TopHat: discovering splice junctions with RNA-Seq. Bioinformatics, 25, 1105-1111. Trapnell, C., et al. (2010) Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol, 28, 511-515. Turečková, V., et al. (2009) Profiling ABA metabolites in Nicotiana tabacum L. leaves by ultra-performance liquid chromatography–electrospray tandem mass spectrometry. Talanta, 80, 390-399. Umen, J.G. and Goodenough, U.W. (2001) Control of cell division by a retinoblastoma protein homolog in Chlamydomonas. Gene Dev, 15, 1652-1661. Vieler, A., et al. (2012) Genome, functional gene annotation, and nuclear transformation of the heterokont oleaginous alga Nannochloropsis oceanica CCMP1779. PLoS Genet, 8, e1003064. Wang, D., et al. (2012) Establishing oleaginous microalgae research models for consolidated bioprocessing of solar energy. Adv Biochem Eng Biotechno, 128, 69-84. Wang, D., et al. (2014) Nannochloropsis genomes reveal evolution of microalgal oleaginous traits. Plos Genetics, DOI: 10.1371/journal.pgen.1004094. Zachleder, V. (1994) The effect of hydroxyurea and fluorodeoxyuridine on cell cycle events in the Chlorococcal alga Scenedesmus quadricauda (Chlorophyta) J Phycol, 30, 274-279.