Functional Groups: Properties & Intermolecular Forces
advertisement

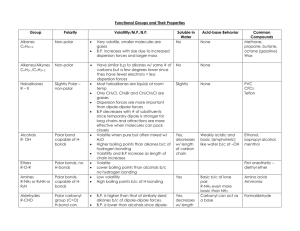
Functional Group Formula Naming Example Types of Intermolecular Forces London dispersion forces only (weak) Melting Point (oC) Hydrocarbons R -ane -ene -yne CH4 = methane Low Halo-alkane R-X (X=halogen) Prefix depends on halogen H3CCl = chloromethane London, dipoledipole Alcohol R-OH –ol H3COH = methanol Aldehydes R-C=O H -al H3C=O = methanal Ketones R-C=O R -one H3C-C=O CH3 =propanone Carboxylic Acids R-C=O OH -oic acid HC=O OH =methanoic acid Boiling Point (oC) Polarity Properties Low Nonpolar Higher than alkane Higher than alkane polar Very low solubility in polar solvents, good non-polar solvents Solubility in water increases as size decreases London, DipoleDipole, Hydrogen Greater than alkanes and halo-alkanes Greater than alkanes and halo-alkanes OH group is polar London, D-D, can accept Hydrogen from H2O London, D-D, can accept Hydrogen from H2O London, D-D. Hydrogen Lower than alcohol Lower than alcohol Polar Lower than alcohol Lower than alcohol polar Higher than alcohol Higher than alcohol Polar Solubility in water increases as size decreases, Ideal solvents (dissolved both polar and nonpolar) Solubility in waterincreases as size decreases Weak acid, conductor Acidic, Volatile-smell Esters R-C =O OR Ethers R-O-R Amines R-N-R R Amides R-C=O N-R R -oate -C attached to O is side chain -parent chain attached to =O -oxy (on smaller chain) -longer chain is parent -amine -longest chain is parent -other 2 are side chains -amide -longest chain with =O is parent -other 2 are side chains H2C-C=O O-CH3 =methyl ethanoate London, D-D, can accept hydrogen from H2O Lower than alcohol and Acid Lower than alcohol and Acid Somewhat Polar Solubility in water increases as size decreases H3C-O-C H3 Propoxypropane London, D-D, can accept hydrogen from H2O Lower than alcohol, greater than hydrocarbon Lower than alcohol, greater than hydrocarbon Somewhat Polar H3C-N-H H =methanamine London, D-D, primary and secondary can hydrogen bond, Less than alcohols, higher than those that can’t h-bond Less than alcohols, higher than those that can’t h-bond Polar Smaller are soluble in water C-C=O N-H H ethanamide London, D-D, primary and secondary can Hydrogen Higher than alcohols but less than acid Higher than alcohols but less than acid Polar Small are very soluble in water MELTING AND BOILING POINT FROM HIGHEST TO LOWEST Amide > Form 2 Hbonds (primary amine has 2 NH groups), And accept Hbonds on both O and N and have 2 Carboxylic Acid > H-bond and 2 oxygens Alcohol > Amine> H-bond, Only 1 oxygen H-bond, Dipoledipole (N weaker than O) Ketone/Al dehyde> Dipoledipole, can’t do Hbonding but can accept Hbonds Ester> Ether> No Hbonding, Dipole-dipole (2 oxygens) No Hbonding, weak polarity, weak dipoledipole (1 oxygen) Haloalkane> Polar, weak dipole dipole (except F>O) Alkane Non-polar, only London dispersion forces INTERMOLECULAR FORCES (between two molecules) FROM STRONGEST TO WEAKEST Hydrogen Bonding (H-bond) > Dipole-Dipole bonds (D-D) > Strongest intermolecular forces. Strong intermolecular force. Occurs with a hydrogen bonded to an O, N or F (most EN elements) Occurs between molecules that have a polar dipole. Creates a very strong dipole To have a polar dipole, the EN difference has to be between 0.4-1.7 and the molecule’s VSEPR shape cannot be symmetrical (dipoles cannot cancel out). Molecules with an O, N and F bonded to an H can form H-bonds with themselves (very strong intermolecular forces leading to higher melting and boiling points). They can also form H-bonds with water, making them very soluble. London Dispersion Forces/Van der Waals forces Between EVERY molecule. Weak forces that occur due to constant the movement of electrons creating flickering/momentary dipoles. The larger the molecule, the greater than force. Molecules with a highly EN atom that has a lone pair (O, N, F) and is bonded to a carbon but not a hydrogen cannot Hbond with themselves but can H-bond with water by accepting the positively charge H atom in water on their lone pair, making them highly soluble.