Photophysics and hydrolytic stability of betalains in aqueous

1 SUPPORTING INFORMATION
2
Photophysics and hydrolytic stability of betalains in
3
aqueous trifluoroethanol
8
9
6
7
4
5
Fernando H. Bartoloni ● Letícia Christina P. Gonçalves ● Ana Clara
B. Rodrigues ● Felipe Augusto Dörr ● Ernani Pinto ● Erick L. Bastos*
SUPPLEMENTARY METHODS
Purification of betanin[1]
10 Beetroots ( Beta vulgaris subsp. vulgaris var. vulgaris, 0.5 kg) were
11 peeled, sliced and homogenized in a centrifugal juice extractor (Phillips–
12
Walita, RI1858) at maximum speed. The juice was centrifuged (1370 × g,
13 30 min, 25 o C), filtered (Whatman qualitative filter paper, grade 4) and the
14 supernatant was stored at –20 o C and used within 5 days. The
15 betanin/isobetanin mixture was purified from beetroot juice by reversed-
16 phase column chromatography (silica gel 90 C18-RP (20 g) conditioned
17 and eluted with deionized water at a flow rate of 0.3 mL min
–1
). The
18 concentration of betanin was determined by assuming a molar absorption
19 coefficient ( ε ) of 6.5 × 10 4 L mol
–1
cm
–1
at 536 nm.
20 Betalamic acid
21 Betalamic acid was obtained from the alkaline hydrolysis of betanin,
22 as described previously.[2]
S1
1 UV-Vis: λ abs max = 430 nm (water, pH 11), ε 424 nm = 3.0 × 10 4 L mol
–1
cm
–1
2 (water, pH 11)[3];
ε 430 nm = 2.65 × 10 4 L mol
–1
cm
–1
(water, pH 11.4).
3 Fluorescence: λ
Fl max = 500 nm (water, pH 11, λ exc
= 420 nm).
4 Indicaxanthin (BtP)
5 Was prepared using a procedure adapted from Schliemann et al.
.[4]
6 L-Proline (100 equiv.) was added to an aqueous solution of betalamic acid
7 (0.5 – 1.0 mL, pH 10) and the resulting solution was stirred at rt. The
8 reaction was monitored spectrophotometrically by the depletion of the HBt
9 absorption band at 430 nm and the concomitant appearance of the BtP
10 band at 485 nm. After completion (ca. 30 min), the solution was cooled (0
11 o C) and HOAc ( conc.
) was added slowly until pH 5. The resulting solution
12 was then stirred at rt for 1 hour. The product was purified through reversed-
13 phase column chromatography (C18 silica gel, water as eluent) and
14 fractions containing BtP were combined and lyophilized. The resulting
15 orange powder was stored at −20 °C and its purity was checked by RP-
16
HPLC analysis prior to use. In a typical run, 0.7 mg (2 μmol) of
BtP were
17 obtained from 0.5 mg (2.5 μmol) of betalamic acid; 54% purified yield.
18 HRMS ( m/z ): [M + H] + calcd. for C
14
H
17
N
2
O
6
+ , 309.1087; found: 309.1082
19 (dif.: 1.62 ppm).
20 LC-ESI(+)-MS: R t
= 6.2 min, ( m / z ) [M + H] + found 309.1.
21 UV/Vis: λ max
485 nm (water), ε 485 nm = 4.8 × 10 4 L mol
–1
cm
–1
(water).[3,5]
S2
1 Fluorescence : λ max fl 520 nm (water, λ exc
= 510 nm), Φ
Fl
= (4.6 ± 0.2) × 10
–3
2 (water, pH = 6) vs.
fluorescein.
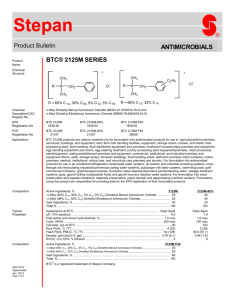
3 BtC
4 7-Amino-3-methylcoumarin (100 equiv.) was added to an aqueous
5 solution of betalamic acid (0.7 mg (3.2 μmol) in 1.0 mL, pH 10) and the
6 resultant solution was submitted to ultrasonic irradiation for 5 min and
7 stirred at rt for an additional 30 min. The reaction was monitored
8 spectrophotometrically by the appearance of the BtC band at 520 nm. After
9 completion, the solution was cooled (0 o C) and HCl ( conc.
) was added
10 slowly until pH 3. The resulting solution was then stirred at rt in the dark
11 for 2 hours. The product was purified through gel-permeation
12 chromatography on lipophilic Sephadex LH-20 with water as eluent. The
13 fractions containing BtC were combined and lyophilized. The resulting
14 purple powder was stored at −20 °C and its purity was checked by RP-
15
HPLC analysis prior to use. In a typical run, 0.4 mg (1 μmol) of
BtC were
16 obtained from 0.7 mg (3.2 μmol) of betalamic acid; 35% purified yield.
17 Complete synthetic procedure and characterization will be reported
18 elsewhere.
19 LC-ESI(+)-MS: R t
= 5.6 min, ( m / z ) [M + H] + found, 369.1.
20 HRMS ( m/z ): [M + H] + calcd. for C
19
H
17
N
2
O
6
+ , 369.1078; found, 369.1082
21 (dif.: –1.08 ppm).
S3
1 UV/Vis: λ max
520 nm (water), ε 520 nm = 6.6 × 10 4 L mol
–1
cm
–1
(water).
2 Fluorescence
: λ max fl 570 nm (water, λ exc
510 nm), Φ
Fl
= (4.3 ± 0.3) × 10
–3
3 (water) vs.
fluorescein.
4 Quantum chemical calculations.
Gaussian09 was used for all quantum-
5 chemical calculations.[6] All structures were optimized in the gas phase at
6 the cam-B3LYP/6-31+G(d,p) level.[7] Stationary points were characterized
7 as minima by vibrational analysis.
8
9 Table S1.
Refractive index ( n ) of water, TFE and their binary mixtures.
10
11
12
13
14
15
TFE (% v/v
) χ
TFE a n b
0
20
0.00 1.333
0.06 1.330
40
60
80
100
0.14 1.322
0.26 1.313
0.49 1.303
1.00 1.291 a Determined considering MW (TFE) = 104.05 g mol
–1
, MW (W) = 18.015 g mol d (TFE) = 1.325 g mL
–1
, d (W) = 0.998 g mL
–1
;
b
–1
,
Determined at 20 ºC using a
Bellingham & Stanley Abbe 60/ED refractometer equipped with a sodium lamp (589.3 nm).
S4
1
2 Figure S1.
Effect of TFE on the absorption and fluorescence spectra of Bn ,
3 BtP and BtC . [ Bn ] = 2.8 μmol L –1
; [ BtC ] = 2.8 μmol L –1
; [ BtP ] = 2.3
4 μmol L –1
.
5
6
S5
1
2 Figure S2.
Normalized fluorescence spectra of fluorescein (standard), BtP
3 and BtC obtained in water using solutions with different optical densities.
4
5
6 Figure S3.
Area under the fluorescence emission curve of fluorescein
7 (standard), BtP and BtC obtained in water using solutions with different
8 optical densities. Linear regression analysis: fluorescein: AUC = (597350 ±
9 746)Abs (Adj-R 2 = 0.9992); BtP
: AUC = (3478 ± 165)Abs (Adj-R 2 =
10 0.9976); BtC : AUC = (3251 ± 108)Abs (Adj-R 2 = 0.9993).
S6
1
2 Figure S4.
Absorption profile of Bn , BtP and BtC in water or in TFE
3 acquired immediately after solution preparation, and at least 8 days later.
4
S7
1
2 Scheme S1
3 References
4 1. Goncalves LCP, Trassi MAD, Lopes NB, Dorr FA, dos Santos MT,
5 Baader WJ, Oliveira VX, Bastos EL (2012) Food Chem 131:231
6 2. Gandia-Herrero F, Garcia-Carmona F, Escribano J (2006) Phytochem
7 Anal 17:262
8 3. Stintzing FC, Herbach KM, Mosshammer MR, Carle R, Yi WG,
9 Sellappan S, Akoh CC, Bunch R, Felker P (2005) J Agric Food Chem
10 53:442
11 4. Schliemann W, Kobayashi N, Strack D (1999) Plant Physiol 119:1217
12 5. Girod PA, Zryd JP (1991) Phytochemistry 30:169
S8
1 6. Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA,
2 Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA,
3 Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J,
4 Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R,
5 Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T,
6 Montgomery JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E,
7 Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K,
8 Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM,
9 Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts
10 R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski
11 JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P,
12 Dannenberg JJ, Dapprich S, Daniels AD, Farkas, Foresman JB, Ortiz JV,
13 Cioslowski J, Fox DJ (2009) Gaussian 09, Revision B.01. Wallingford CT.
14 7. Perdew JP, Burke K, Ernzerhof M (1996) Phys Rev Lett 77:3865
15
S9