Module 2b – Atoms & Reactions
advertisement
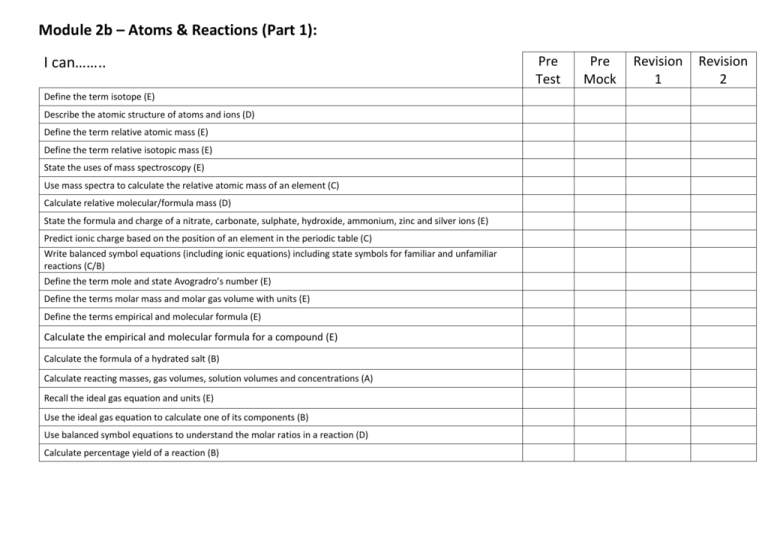
Module 2b – Atoms & Reactions (Part 1): I can…….. Define the term isotope (E) Describe the atomic structure of atoms and ions (D) Define the term relative atomic mass (E) Define the term relative isotopic mass (E) State the uses of mass spectroscopy (E) Use mass spectra to calculate the relative atomic mass of an element (C) Calculate relative molecular/formula mass (D) State the formula and charge of a nitrate, carbonate, sulphate, hydroxide, ammonium, zinc and silver ions (E) Predict ionic charge based on the position of an element in the periodic table (C) Write balanced symbol equations (including ionic equations) including state symbols for familiar and unfamiliar reactions (C/B) Define the term mole and state Avogradro’s number (E) Define the terms molar mass and molar gas volume with units (E) Define the terms empirical and molecular formula (E) Calculate the empirical and molecular formula for a compound (E) Calculate the formula of a hydrated salt (B) Calculate reacting masses, gas volumes, solution volumes and concentrations (A) Recall the ideal gas equation and units (E) Use the ideal gas equation to calculate one of its components (B) Use balanced symbol equations to understand the molar ratios in a reaction (D) Calculate percentage yield of a reaction (B) Pre Test Pre Mock Revision 1 Revision 2 Module 2b – Atoms & Reactions (Part 2): I can…….. Calculate the atom economy of a reaction (C) State the benefits of developing processes that have a high atom economy (D) State the formulae for hydrochloric, sulphuric, nitric and ethanoic acid (E) State the formulae for sodium hydroxide, potassium hydroxide and ammonia (E) Define the term acid and alkali (E) Define term strong and weak acid (E) Write word and symbol equations for reactions between acids and bases (carbonates, metal oxides, alkalis) (C/B) Write word and symbol equations for the reactions between acids and metals (C/B) Write an ionic equation for a reaction between an acid and alkali (D) Describe how to prepare a standard solution (C) Describe how to carry out a titration (C) Use titration data to calculate unknowns (A) Recall the rules for assigning oxidation numbers (E) Use oxidation number rules for calculating oxidation numbers (D) Use roman numerals to indicate the oxidation number of an element that has different oxidation numbers (E) Define the terms oxidation and reduction (in terms of electrons and oxidation number) (E) Interpret redox equations to identify reduction and oxidation (B) Pre Test Pre Mock Revision 1 Revision 2