Documents_files/Chp 1 Notes CChem
advertisement

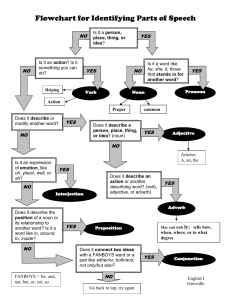
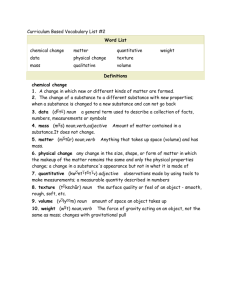
Chapter 1 – Chemistry: The Science of Matter I. Composition, Structure, and Behavior A. Chemistry (noun) - the science that investigates and explains the structure and properties of matter. B. Matter (noun) 1. Mass (noun) 2. Volume (noun) - C. Properties of matter describe the D. Examining Matter 1. Macroscopic (adjective) - 2. Submicroscopic (adjective) - II. Classifying Matter A. Classification by composition – What is it made of? How much of different elements are present? 1. Qualitative (adjective) - 2. Quantitative (adjective) - 1 B. Mixture (noun) – combination of two or more types of matter; basic identity is not changed 1. Mixtures can be separated by physical changes, which takes advantage of physical properties. a. physical changes (noun) – a change in matter that does not involve a change in the identity of individual substances. Examples: b. physical properties (noun) – characteristics of matter shown without changing the identity. Examples: 2. Types of mixtures a. heterogeneous (adjective) – b. homogeneous (adjective) - i. Type of homogeneous mixture is a solution. SoluteSolventExample- sugar water C. Pure Substances – Every piece is the same substance, having a fixed composition and properties. 1. Different from mixtures in two ways a. Every sample has the exact same chemical and physical properties b. Every sample is made of the exact same substance(s) 2 2. Types of Pure Substances a. Elements (noun) – made of only one kind of atom, only one type of substance, cannot be broken down into simpler substances. Examples: b. Compounds (noun) - a combination of two or more different elements joined together by a chemical bond in a fixed ratio Examples: III. Identifying Matter by its Properties A. States of matter (noun) B. Density (noun) – IV. Chemical Properties and Changes A. Chemical properties (noun) – Examples: B. Chemical changes (noun) – also known as a chemical reaction, 1. Chemical change involves energy change. a. Exothermic (adjective) – b. Endothermic (adjective) – 3 Academic Words Identify – group 11 Composition Involve Structure Behavior Investigate Explain Characteristic – group 10 Illustrate – group 10 Demonstrate – group 10 Analyze – group 4 Procedure – 14 Observe – 4 Domain Specific Words Chemistry Matter Mass Volume Macroscopic Submicroscopic Qualitative Quantitative Mixture Physical change Physical property Homogeneous Heterogeneous Solution Element Compound Density Chemical change Chemical property Exothermic Endothermic 4