6.3 Activity - Lipids models.doc
advertisement

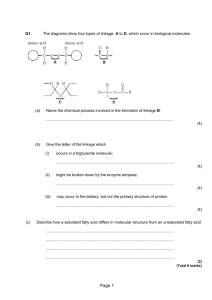
The Chemistry of Lipids - Fats Name ________________________ Period _____ Date ______________ Lipids are fats, oils, and waxes. ● Fats contain carbon, hydrogen and oxygen, but the proportion of hydrogen is not the same as in carbohydrates. ● Because lipids contain very little oxygen, they can yield large amounts of energy when combined with oxygen. ● With this lab exercise you will be expected to: 1. Understand the structure of alcohols and fatty acids 2. Construct molecular models of fat 3. Distinguish between models and actual chemical formulas of fat 4. Determine the molecular characteristics of fats Procedure: Students will work in groups no larger than 2 Each student will be responsible for filling out his/her own worksheet. Each group needs: 15 (carbon – C) = black pieces 9 (oxygen – O) = red pieces 32 (hydrogen – H) = yellow pieces 50 wood bonds (used for single bonds) 6 spring bonds (used for double bonds) Fats On a molecular basis, all fats are somewhat similar. ● Just as carbohydrates are composed of smaller molecules called monosaccharides, all fats are composed of smaller molecules. ● The smaller molecules in fats are glycerol and fatty acids. ● Glycerol (Make 1 of this molecule) a. What elements are present in glycerol? ______________________________________ 1 b. Are there any elements in glycerol not present in carbohydrates? __________________ C c. What is the molecular formula for glycerol? (how many of each) H O d. Build a structural model for glycerol. e. What is the ratio of hydrogen atoms to oxygen atoms in glycerol? __________________ f. How does this ratio compare to the ration in carbohydrates? ______________________ Fatty Acids ● acid. The second molecule which contributes to forming fat is a long molecule called a fatty Many different fatty acids exists, but all are similar in many ways. ● All fatty acids have a radical group (R, different in every fatty acid) and a COOH. Therefore we can represent any fatty acid with the general formula of R-COOH ● (Make Butyric acid 3 of this molecule) a. Build butyric acid. b. What is the molecular formula of butyric acid? (how many of each) C H O c. Does a 2:1 ratio of hydrogen atoms to oxygen atoms exist in fatty acids? Forming Fats A fat molecule consists of one glycerol molecule and three fatty acid molecules joined together. a. Attempt to join together the glycerol molecule and three fatty acids. Will the fat molecule stay together? _______________ b. It will be necessary to remove three –OH ends from the glycerol molecule and the –H end from each of the fatty acids’ carboxyl end in order to join the molecules. Does this enable you to join the molecules together? ______________ If so, do it. c. Fatty acid + glycerol → fat + water d. How many water molecules are formed when one fat molecule is produced? ___________ Interpretations 1. What type of reaction is required to build fats? 2. What two types of molecules are necessary to build a fat molecule? 2 The Chemistry of Lipids - Fats Name ANSWER KEY Period _____ Date ______________ Lipids are fats, oils, and waxes. ● Fats contain carbon, hydrogen and oxygen, but the proportion of hydrogen is not the same as in carbohydrates. ● Because lipids contain very little oxygen, they can yield large amounts of energy when combined with oxygen. ● With this lab exercise you will be expected to: 1. Understand the structure of alcohols and fatty acids 2. Construct molecular models of fat 3. Distinguish between models and actual chemical formulas of fat 4. Determine the molecular characteristics of fats Procedure: Students will work in groups no larger than 2. Each student will be responsible for filling out his/her own worksheet. Each group needs: 15 (carbon – C) = black pieces 9 (oxygen – O) = red pieces 32 (hydrogen – H) = yellow pieces 50 wood bonds (used for single bonds) 6 spring bonds (used for double bonds) Fats On a molecular basis, all fats are somewhat similar. ● Just as carbohydrates are composed of smaller molecules called monosaccharides, all fats are composed of smaller molecules. ● The smaller molecules in fats are glycerol and fatty acids. ● Glycerol a. What elements are present in glycerol? Carbon, hydrogen, & oxygen b. Are there any elements in glycerol not present in carbohydrates? No, all the same. 3 c. What is the molecular formula for glycerol? (how many of each) C 3 H 8 O 3 d. Build a structural model for glycerol. e. What is the ratio of hydrogen atoms to oxygen atoms in glycerol? 8 : 3 f. How does this ratio compare to the ration in carbohydrates? Different, carbohydrates have a 2 : 1 ration of hydrogens to oxygens. Fatty Acids ● acid. The second molecule which contributes to forming fat is a long molecule called a fatty Many different fatty acids exists, but all are similar in many ways. ● All fatty acids have a radical group (R, different in every fatty acid) and a COOH. Therefore we can represent al fatty acids with the general formula of R-COOH Butyric acid ● a. Build butyric acid. b. What is the molecular formula of butyric acid? (how many of each) C 4 H c. Does a 2:1 ratio of hydrogen atoms to oxygen atoms exist in fatty acids? No, 4:1 8 O2 Forming Fats A fat molecule consists of one glycerol molecule and three fatty acid molecules joined together. a. Attempt to join together the glycerol molecule and three fatty acids. Will the fat molecule stay together? No b. It will be necessary to remove three –OH ends from the glycerol molecule and the –H end from each of the fatty acids’ carboxyl end in order to join the molecules. Does this enable you to join the molecules together? Yes If so, do it. c. Fatty acid + glycerol → fat + water d. How many water molecules are formed when one fat molecule is produced? 3 Interpretations 1. What type of reaction is required to build fats? Condensation reaction or dehydration synthesis 2. What two types of molecules are necessary to build a fat molecule? A glycerol and fatty acids 4