U1 Project
advertisement

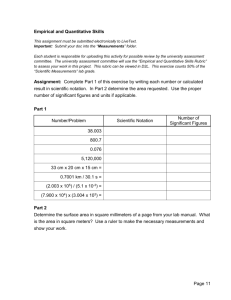
Name ____________________________ Period __________ Unit 1 Scientist’s Tools Mini Project Purpose: To apply the Unit 1 Objectives in a real world manner. Procedure: 1. Locate a quantitative scientific fact that expresses a very large or small amount. It must have units of either length, mass, time, volume, moles, or joules. 2. If it was given in scientific notation, change it to ordinary notation. If it was given to you in ordinary notation, change it into scientific notation. 3. Find a picture that relates to the scientific fact. 4. Create a word problem converting the unit of your quantitative, scientific fact to a different unit. 5. Solve the problem showing work using dimensional analysis. 6. Express your answer in correct number of significant figures and units. Presentation: See example on back. 1. Place all of the above on a sheet of 8 x 10 copy paper or cardstock paper. It can be colored. 2. The value of scientific fact should be in ordinary notation at the top of the paper with the number in scientific notation in parentheses beneath it. 3. List the scientific fact and follow it with a picture underneath it 4. The word problem you created will be written underneath the picture. 5. Work in dimensional analysis form will follow. 6. Your answer will be boxed in. Rubric on how it will be scored: (50 points) What I’m looking for Quantitative scientific fact Presentation of fact in scientific notation/ordinary notation Related picture Word Problem Showing the work for the word problem in dimensional analysis Answer in proper significant figures and units Creativity & Neatness Possible Points 5 points (Bonus: 5 pts if not duplicated in any of the classes) 10 points 10 points 5 points 10 points 5 points 5 points Earned Points Example (you cannot use this one) .000000000000000000000000000000910938291 kg (9.10938291 × 10-31 kilograms) THE MASS OF AN ELECTRON Picture is to be placed here! Convert the mass of electron in kilograms into grams. 9.10938291x 10-31 kg X 1000g = 1 kg 9.10938291 x 10-28 g