TENTATIVE LABORATORY SCHEDULE (Molecular Biology 2) (1/21
advertisement

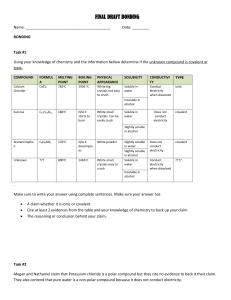
Classification of Mystery Substances This document supports the safety activity “Mystery Substance Identification: The Identification of Unlabeled Chemicals Found on School Premises” from Flinn Scientific. This is available on the Flinn Scientific website at: http://www.flinnsci.com/media/755029/mystery.pdf Background information Your supervisor has just handed you a tray of containers filled with various materials. Scattered on the bottom of the container are the labels that are suppose to be to be on these bottles. ______________________________________________________ List of chemical labels in the box: Mineral Oil Methanol Sodium dodecyl sulfate (SDS) Hydrochloric Acid Hexane Hydrogen peroxide (10%) _______________________________________________________ Your supervisor says she is worried there may be chemicals stored in the tray that are incompatible. She wants you to identify the classes of substances in the tray so that they can be stored properly while arrangements are made with the local chemical waste disposal company. General Classifications of Chemicals: Acid: substance that increases the H+ ion concentration in aqueous solution Base: substance that decreases the H+ ion concentration in aqueous solution Oxidizing agent: substance that causes the oxidation (loss of electrons) of another substance Reducing agent: substance that causes the reduction (gain of electrons) of another substance Organic solvent: liquid made of carbon, usually in combination with hydrogen, oxygen, nitrogen, and/or sulfur Water reactive material: substance that can chemically interact with water usually with a release of heat or light. 1 This lab exercise concerns the characteristics and proper handling of various classes of hazardous materials that may be found in a biotech lab. It is not intended to be training in chemical identification. In fact, if you are presented with this kind of problem in the workplace, you need to consult the Safety Officer for you company or institution. Rather, this lab is a condensed version of the types of procedures that a professional team would undertake when identifying a chemical. The simple tests are designed to assign them to a broad classification for further identification and/or disposal. Pay attention to both positive indicators as well as negative indicators in the testing procedures. General Safety Precautions Materials used in this lab may be hazardous; study the MSDS for each potential mystery substance listed below. Choose one chemical at a time to work with. Wear gloves, coat, and safety glasses. The reactions described are relatively safe. Therefore, unless otherwise directed by the protocol, you may work either at your bench or in the chemical hoods. Cover your work area with bench paper. Laboratory Outline: Day one: 1. Outline experimental protocol for lab 2. Research safety information on unknowns 3. Research chemical characteristics of unknowns 4. Classify unknowns (by chemical class) Day two: 1. Complete testing of unknowns 2. Complete discussion in notebook: Disposal information is available in the Flinn catalog. Laboratory Notebook You should include the following information in your laboratory notebook: Safety information: A table of unknowns and precautions/ hazards for each Chemical characteristics: A table of unknowns and their chemical properties Disposal information: A table of each unknown and disposal method Results: A summary table of each unknown tested, its physical characteristics, and the results of all tests done Discussion: 1. Determination of the probable identity of each unknown, along with the reasoning used to reach this conclusion 2. Plan for how each unknown should be stored until it can be picked up for disposal. 2 APPENDIX: Protocol for identifying unknowns Step 1. Make a preliminary inspection: Check the container. Is it a commercial container? Describe the condition of the container. A primary inspection on the physical characteristics may help in the identification of the material. Has the liquid been prepared in the lab? Does the bottle indicate the age of the material, when it was purchased, when it was made? This information may give an indication of identity. What are the physical characteristics of the material? Solid: powder, crystal, granular, or another description Color: homogeneous or heterogeneous? Check a color chart that gives an indication of the identity of the material (for example, blue crystals may indicate a copper compound or salt). Liquid: color, viscosity Open the container in a fume hood: Is there any indication that the material reacts with the air? If so, the material may be more dangerous than practical to handle. Stop the identification process and call for professional help. Write down all physical characteristics of the unidentified compound. Can any of these physical traits give an indication as to the identity? These characteristics may be needed to backup your conclusions from later tests. Step 2. Exposure to water: Is the material water reactive? Is the material water soluble? TEST: Place about 3 mL of distilled water in a test tube. Add 2 drops of the liquid or about 0.1 g of the solid to the water. Note any reaction. Carefully feel the test tube for any temperature change. Is the reaction violent? Does the reaction generate heat? Is the reaction exothermic or endothermic? (what exothermic reactions are commonly found in the lab?) Label as water reactive. 3 TEST: After testing for water reactivity, mix 1 g of material in 10 mL of water. Look for residue and estimate solubility (insoluble, partially soluble, or soluble) Is the substance water soluble? If yes, then proceed to step 3. Is the substance water insoluble? If yes, and the substance is a liquid then proceed to step 4 to determine density. If yes and the substance is a sold then label as water insoluble and proceed to step 6. Step 3. Check the pH: TEST: With pH indicator strips use a Pasteur pipette to remove a drop of the liquid from the aqueous solution prepared in Step 2. Place on the indicator strip and determine the pH using the scale provided on the box. Record the pH. 1. Is the pH below 3 or above 11? This indicates that the material is a corrosive material and should be labeled as a “Corrosive acid” or “Corrosive base.” 2. A pH between 3 and 5 indicates the following possibilities: 1. Weak acid 2. Dilute strong acid 3. Salt of either a weak base or a strong acid In this case the material can be disposed of by neutralizing the material by titration with a base and disposal down the sewers. 3. A pH between 8 and 11 indicates the following possibilities: a) Weak base b) Dilute strong base c) Salt of either a weak acid or a strong base (Note: With an alkaline pH there is a possibility that the compound is a sulfide or a cyanide compound. If either of these is neutralized there is a possibility that cyanide gas or hydrogen sulfide gas will be generated. To test for these possibilities add dilute calcium chloride (0.1 M) to the solution in the test tube. If a white precipitate forms, you most likely have a phosphate or carbonate compound as a salt. Unless a precipitate forms assume the material is hazardous and do not proceed. Call a hazardous materials specialist. The presence of the precipitate indicates that the material can be neutralized with acid and disposed of through the sewer system.) Step 4. Density: The density of a liquid can be determined by weighing a known volume. Determine the mass of a small weigh boat or use the tare button on the balance. Pipette one mL of the liquid into the weight boat. Record the mass and subtract the mass of the weigh boat. 4 Repeat this more two times and average the mass. Density is recorded in weight per volume (g/mL). a. If the material is water soluble, and is less dense than water it will probably be a low molecular weight alcohol. b. If the material is water soluble, and has a density that is greater than water it may indicate a concentrated acid. c. If the liquid is not water soluble, and is less dense than water, it may be: A hydrocarbon (hexane, pentane) Fat (cooking oil, mineral oil) High molecular weight alcohol or amine d. If the liquid is not water soluble, and is more dense than water, it is likely to be a halogenated hydrocarbon (chloroform). Step 5. Oxidizing or reducing agent: a. For a water-soluble material lower the pH to 5-7 with a small quantity of acid. Add a drop of the pH adjusted solution to about 1 mL of an aqueous 1 M potassium iodide solution. If a yellow color develops, then label the material as a water soluble oxidizing agent. b. Prepare a solution of the material at a concentration of 50 mg / mL. Add the solution drop wise to a dilute 0.1 mM potassium permanganate solution. If the potassium permanganate solution changes from a pale pink color to clear, then label the material as a reducing agent. Step 6. Check for flammability: a. Dip a glass rod into the liquid material and expose to an open flame. b. Take a small piece of solid material and expose to an open flame. c. If a flame develops then label as Flammable. Also note the color of the flame. 5