Letter of Intent ()
advertisement

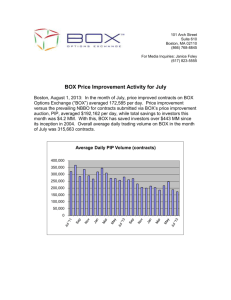
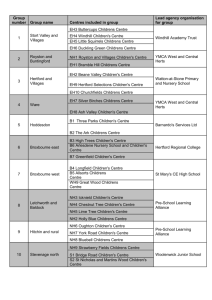
Technology Development Fund (TDF) POCG/TDG Letter of Intent The proof of concept grant (POCG) is awarded annually and provides up to $50,000 to advance early stage technologies in the validation or proof of concept phase. The technology development grant (TDG) is awarded annually and can provide up to $150,000 to advance later stage technologies, where the concept has been proven. The awards can be used for prototype development, medicinal chemistry; pharmacokinetic/ pharmacodynamic or animal efficacy studies for potential therapeutics, software optimization, human clinical data, other advanced pre-commercial research. Approximately 70% of the award will be spent through outside contract research organizations (CROs) who will work with the principal investigator and TIDO to further develop the technology. The TDF eligibility criteria are listed in the “TDF policies and guidelines” found on [link]. Please address the following industry-specific questions while answering “Stage of development”: Health care IT 1. Is there an existing software code for your proposed technology? Has it been tested? 2. Is there a similar software or product (on the market or in development) that performs the same tasks? Which one? How is your proposed technology different from what is currently available? 3. Does the software need to integrate with BCH’s existing IT infrastructure? 4. What are your plans for testing the software to be developed? Do you have departmental IT support (for communication with the developers and ISD)? Is there departmental interest in launching the software internally upon completion? Devices 1. Do you currently have a prototype? If so, what type (drawings, non-functional model, functional/working)? 2. Was your prototype tested? If yes, how was it tested (ex vivo/in vivo)? Therapeutics 1. Do you have novel compounds? 2. If so, are these acting on novel or known targets? 3. Is mechanism of action of compound known? 4. If you do not have compounds, do you have a novel target/pathway? How was your target/pathway validated for the proposed indication? Diagnostics 1. Is your technology a novel biomarker? 2. On what platform(s) was it validated? 3. How many samples have you collected? 4. How many samples have you tested? What were your positive/negative controls? Technology Development Fund| Boston Children’s Hospital | 300 Longwood Avenue | Boston MA 02115 Phone (617) 919-3019 | Fax (617) 919-3031 | Technology.Development@Childrens.Harvard.edu Please use Arial 11-point font for the LOI form below. Project Title Principle Investigator Name Title Department Phone Email Application Type TIDO/IPO CMCC Number Case Manager TDG – POCG (please circle one) Please check: Are there existing or predicted contractual obligations around this technology, such as through license, option, or MTA? Yes No Product: In a few words, describe the product concept. What is the industry subsector (HCIT, device, therapeutic, diagnostic, other)? What is the target patient population/disease area? Medical Need: Briefly describe the: 1) Medical need, 2) Current standard of care and currently available products, 3) Limitations of current approaches and 4) Anticipated impact of proposed product on patient care. Will your product change the clinical workflow, and if so, how? Technology Development Fund| Boston Children’s Hospital | 300 Longwood Avenue | Boston MA 02115 Phone (617) 919-3019 | Fax (617) 919-3031 | Technology.Development@Childrens.Harvard.edu Commercial Potential: 1) What portion of the population affected by the disease could this product help? 2) What are potential product development obstacles (regulatory, reimbursement, technical, competition)? 3) Who makes the purchasing decisions (hospitals, patients, etc.)? Stage of Development: Refer to the questions on page 1 for the relevant industry subsector (therapeutic, medical device, diagnostic, HCIT) for your technology. If “other”, please elaborate on the stage of development. In addition, please include your most relevant data to support the stage of development. Technology Development Fund| Boston Children’s Hospital | 300 Longwood Avenue | Boston MA 02115 Phone (617) 919-3019 | Fax (617) 919-3031 | Technology.Development@Childrens.Harvard.edu Project Objectives: Specific aims, desired outcomes, long-term goals Team: 1) BCH investigators and relevant experience 2) External partner/CRO: if the CRO has not been identified yet, please specify which aspects of the project will be outsourced 3) If you currently have (or anticipate needing) collaborators, please identify them and their institutions. Requested funds and other resources: 1) Estimated funds needed to complete the project objectives 2) Prior, current, and pending sources of support for proposed project (FTE/space/ funding/internal resources) Technology Development Fund| Boston Children’s Hospital | 300 Longwood Avenue | Boston MA 02115 Phone (617) 919-3019 | Fax (617) 919-3031 | Technology.Development@Childrens.Harvard.edu References: publications (including your own data) that should be considered in the evaluation and/or experts that should be consulted to validate technology For any questions, please contact Carla Haslauer at technology.development@childrens.harvard.edu or 617-919-1375 (4-1375). Technology Development Fund| Boston Children’s Hospital | 300 Longwood Avenue | Boston MA 02115 Phone (617) 919-3019 | Fax (617) 919-3031 | Technology.Development@Childrens.Harvard.edu