Achievement Scale
advertisement

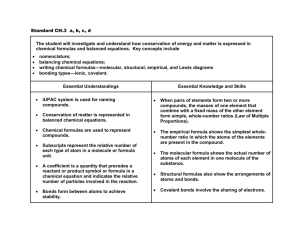
Achievement Scale Content Area: Physical Science Grade Level: 9 Unit: Chemical Bonding Learning Goals: I will understand why and how elements join to form molecules and compounds. I will predict how elements bond based on their electronic structure/location in the Periodic Table. I will be able to explain chemical reactions in terms of the Law of Conservation of Mass. Score 4: Student demonstrates in-depth inferences and applications of the learning goal(s) and can reconstruct and apply their knowledge from limited information: The student: Can explain the law of conservation of mass and how this law applies to chemical reactions. Can use the Law of Conservation of Mass to balance chemical reactions. Can explain, using the terms valence electrons and Octet Rule an atoms oxidation number. Can analyze the ratios in chemical equations to predict the amounts of products formed or reactants needed for the reaction to take place. Can write chemical formulas for compounds when given the name of the compound. Can compare and contrast endothermic and exothermic chemical reactions. Score 3: Student demonstrates no major errors or omissions regarding the learning goal(s) that were explicitly taught: The student: Can identify the type of bond that will be formed between atoms when given two specific atoms. Can write formulas for chemical compounds based on their oxidation numbers. Can state whether the Law of Conservation of Mass is obeyed in a given chemical reaction. Can identify a written chemical reaction as either endothermic or exothermic. Can write chemical formulas for compounds when given the atoms that are bonded together. Can name compounds for chemical formulas without the use of a dichotomous key. Score 2: The student demonstrates no major errors or omissions regarding the simpler details and processes that support the learning goal(s). The student : Can define the term oxidation number and predict an atoms oxidation number. Can define the term ion and predict the charge of ions based on an atoms location in the periodic table. Can define the terms ionic, covalent and metallic bond. Can identify subscripts and coefficients and determine how many atoms there are in a given chemical compound. Can write chemical formulas for compounds when given the ratio of atoms that are bonded together. Can define and identify products and reactants in a chemical equation. Can state what makes a chemical reaction endothermic or exothermic. Can name compounds for chemical formulas when given the dichotomous key. Can define and identify physical and chemical changes. Score 1: With help (being given word banks, manipulated equations, retakes), the student demonstrates a partial understanding of the simpler details and processes that support the learning goal(s) stated for a Score of 2. Score 0: Even with help, no success Score 4 Example Assessment Items: Describe an experiment that would allow you to determine whether the Law of Conservation of Mass was obeyed when you mixed 2 chemicals. Academic Vocabulary: Oxidation number Ions Law of Conservation of Mass State the types of reactions seen below and explain the difference. Ionic Bond Covalent Bond If element Y has 1 valence electron and element X has 6 valence electrons, what is the most likely formula of a compound forming between element Y and X? Metallic Bond Score 3 Example Assessment Items: Products What is the formula for the compound magnesium nitride? Reactants Chemical Reaction Endothermic What type of bond forms between Na and Cl? What type of bond forms between Nitrogen and Oxygen? Score 2 Example Assessment Items Elements in the first column of the periodic table are known as alkali metals. These elements, when ionized, have an oxidation number of ___. Exothermic Chemical Formulas Subscripts Coefficients Covalent bonding occurs when ___________________. Identify the products in the reaction: CH4 + O2 CO2 + H2O District Mission: Every student. Every day. District Vision: A promise of learning, dignity, and respect for all.