File - Tompkins101.com
advertisement

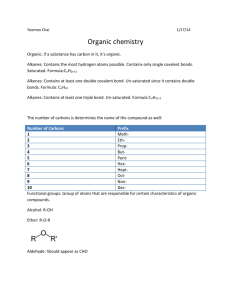
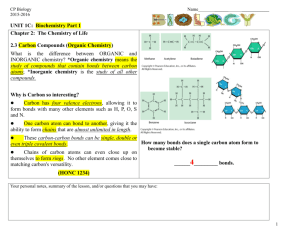
Organic vs. Inorganic 9/26/2012 7:04:00 PM Reminder: Ionic = conductors Covalent = non-conductors Classifying Compounds 1) Inorganic Compounds 2)Organic Compounds 2. What do you think organic means? - In chemistry it means that all compounds contain carbon o The compounds of living things This is how it is going to relate to Biology when we get there o So, another way to think of it: anything that is living, contains carbon in it - Exceptions: CO2 and CO are not living things so are not considered organic (what are these called again? Carbon dioxide and carbon monoxide) o Does anyone know where carbon monoxide come from? It can kill you, so you should know! It will come from exhaust fumes, but it can also come from cooking… normally your red blood cells will grab onto oxygen and use that to feed your body, but if there’s no oxygen (too much CO) in the air your red blood cells will grab onto it and your brain can’t use it so you will go to sleep and never wake up. 1. Inorganic Compounds - Deals with all the ionic compounds we’ve been dealing with - Also includes covalent compounds without carbon o Ex: NH3 (ammonium) - EXCEPTION: the covalent compounds CO2 and CO (even though they contain C, they are not living organisms) Organic? : C2H6? Yes NH3? No CH4O? yes 2A. Hydrocarbons What elements do you think are in this? - These are simply compounds that contain H and C (ONLY) - They are all combustible! (They burn) o Example: fossil fuels… what do you think fossil fuels are? Coal = fuel that gets produced when plants and animals die and gets compressed… it’s a dirty fuel Oil = the difference is how compressed it is… if it is really compressed, it’s coal, if it’s not as compressed, it’s oil Natural gas = a little bit compressed - Carbon footprint: how much carbon you release… CO2 builds up between the earth and the ozone layer… so when heat comes from the sun it should bounce off (reflect off) the earth… but the CO2 traps it and heats the earth up 2B. Other organic compounds - Example: alcohols, sugars, etc 1. Example: C2H6 2. Classifying: - organic - Covalent - Hydrocarbon 3. Lewis covalent diagram - Start with the carbons… draw the valence e- Then draw the carbon-carbon bond - Then draw the H’s with their valence e- Then draw the carbon-hydrogen bonds 4. Structural formula - Draw the C’s and H’s with just the bonds (lines) 5. Space filling model - Write the C’s and H’s… draw a bubble around the two C’s and then lumps where the H’s attach Example: NH3 Classify: inorganic, covalent Lewis Diagram: N first (with e-), then the H’s (with e-) then the bonds Structural: just the N and the H’s with the bonds (lines) Space filling: N in a circle, H’s and lumps added on Example: CH4O Classify: organic, covalent… is it a hydrocarbon? NO! Lewis: C (with e-), O (with e-) and add the H’s (with e-). Then draw bonds (lines) Structural: C, O, H’s with the bonds (lines) Space filling: C in a circle, O attached to the C, add the H’s as lumps Homework: organic vs. inorganic worksheet 9/26/2012 7:04:00 PM 9/26/2012 7:04:00 PM