Chemistry
advertisement

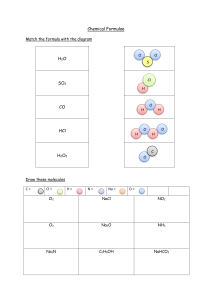
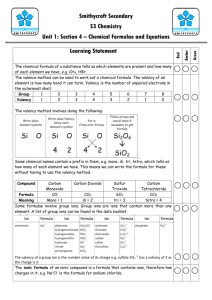
Winter Vacation Homework 2015-16 Class – VIII Chemistry Worksheet 1 1. Balance the following equations --------------------i) Zn + NaOH Na2ZnO2 + H2 ii) Pb(NO3)2 PbO + NO2 + O2 iii) Mg + SO2 MgO + S iv) NH3+ O2 H2O + N2 v) Fe + HCl FeCl2 + H2 2. Differentiate between the following ----------------i) Oxidation and Reduction ii) Cation and Anion iii) Acid and Base iv) Diamond and Graphite v) Combination reaction and decomposition reaction 3. Make a 10-15 pages project on ‘Metals and Non-metals and their applications’ in a project file. Paste the samples or pictures also. 4. Learn Valency chart and derive 20 Chemical formulae from your syllabus in a separate middle page of a file. ****************** Winter Vacation Homework 2015-16 Class – VIII Chemistry Worksheet 2 1. Balance at least 15 chemical equations from chapters 4 and 5. 2. Balance the following equations and also indicate the type of reactions taking place------(Catalytic, Thermal Decomposition, Combination, Simple Dispalcement, Double Decomposition, Reversible, Thermal dissociation) ∆ i) N2O4 NO2 ii) Pb(NO3)2 + HCl PbCl2 + HNO3 iii) HNO3 H2O + NO2 + O2 Pt iv) NH3 + O2 NO + H2Os 700 – 8000C 3. Write the chemical formulae for the following compounds----------i) Calcium Nitride ii) Aluminium Sulphate’ iii) Sodium Bromide iv) Magnesium Chloride v) Lead (II) Nitrate vi) Silver(I) Oxide vii) Mercuric oxide viii) Potassium dichromate ix) Copper Suphide x) Sulphuric Acid 4. Give two uses of the following ---------------------i) Hydrogen iv) Oxygen ii) Acids v) Base iii) Nitrogen 5. An element has 5 protons and 6 neutrons in its nucleus. Calculate its atomic number and mass number. Name the element state its valency. Write the formulae of its nitrate and chloride. ********************** Winter Vacation Homework 2015-16 Class – VIII Chemistry Worksheet 3 1. Write the formulae of following compounds ------i) Calcium Nitrate ii) Sodium Carbonate iv) Potassium oxide v) Acetic Acid vii) Zinc Sulphide viii) Sulphuric Acid x) Magnesium bisulphate iii) Sodium Suphate vi) Ammonium Chloride ix) Silver Nitrate 2. Complete and balance the following chemical equations ----------i) Ca + ii) CH4 + iii) C + (red hot Coke) iv) Mg + v) Na + H2O vi) KClO3 H2SO4 O2 H2O steam H2O 3. Name the following in the given equation--------------ZnO + C Zn + CO a. The substance oxidised. c. The oxidising agent b. The substance reduced. d. The reducing agent. 4. State the type of change in the following ---------i) iii) Rusting of iron Growing of a plant iv) Water Cycle ii) iv) Burning of Candle Falling of leaves from trees 5. Complete the table pertaining to the long form of the periodic table-------------Element Atomic Number Hydrogen Electronic Configuration Mass No. 1 Neutrons 6 Oxygen Sulphur Chlorine Calcium 16 32 35 20 ****************** Metallic/ Non Valency –Metallic