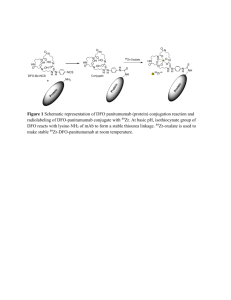
Total synthesis of a functional SUMO
advertisement

Total synthesis of a functional SUMO-1 peptide conjugate. Nathalie Ollivier, Emmanuelle Boll, Hervé Drobecq, Laurent Raibaut, Jérôme Vicogne* and Oleg Melnyk. UMR CNRS 8161 CNRS, Université de Lille, Institut Pasteur de Lille, 1 rue du Pr Calmette, 59021 Lille Cedex, France. The large diversity of chemical methods available now for producing modified peptides in high purity is increasingly used for the total or semisynthesis of modified proteins which cannot be easily produced using biological methods. Proteins featuring well-defined post-translational modifications at specific sites such as ubiquitylation belong to this family. The total synthesis of small ubiquitin-like modifier (SUMO) proteins and SUMO conjugates is challenging and is addressed in this work. Indeed, we describe for the first time the total synthesis of a folded, functional and reversible 12.1 kD SUMO-1 conjugate (Figure),1-2 which was assembled using a onepot N-to-C three segments assembly process relying on sequential NCL and SEA ligation reactions.3-6 The assembly of SUMO-1 proteins required to push the limits of current technology by addressing the synthesis of long (~ 50 AA) SEA peptides and peptide thioesters. This was achieved by developing a novel SEA PEG-based solid support which is compatible with standard Fmoc-SPPS. The tertiary structure and functionality of SUMO-1 modifier within the conjugate as well as its reversible nature was demonstrated by the total and specific cleavage of the conjugate with a recombinant SUMO-1 deconjugating enzyme. MALDI-TOF RP-HPLC 1. 2. 3. 4. 5. 6. E. Boll, H. Drobecq, N. Ollivier, L. Raibaut, R. Desmet, J. Vicogne and O. Melnyk, Chem. Sci., 2014, 5, 2017-2022. E. Boll, H. Drobecq, N. Ollivier, A. Blanpain, L. Raibaut, R. Desmet, J. Vicogne and O. Melnyk, Nat. Protocols, 2015, 10, 269-292. N. Ollivier, J. Dheur, R. Mhidia, A. Blanpain, O. Melnyk, Org. Lett., 2010, 12, 5238 (2010) N. Ollivier, J. Vicogne, A. Vallin, H. Drobecq, R. Desmet, O. El-Mahdi, B. Leclercq, G. Goormachtigh, V. Fafeur and O. Melnyk, Angew. Chem., Int. Ed., 2012, 51, 209-213. L. Raibaut, N. Ollivier, O. Melnyk, Chem. Soc. Rev., 2012, 41, 7001. L. Raibaut, J. Vicogne, B. Leclercq, H. Drobecq, R. Desmet, O. Melnyk, Bioorg. Med. Chem., 2013, 21, 3486.