Figure 1, Figure 2, Figure 3, Figure 4, Figure 5
advertisement
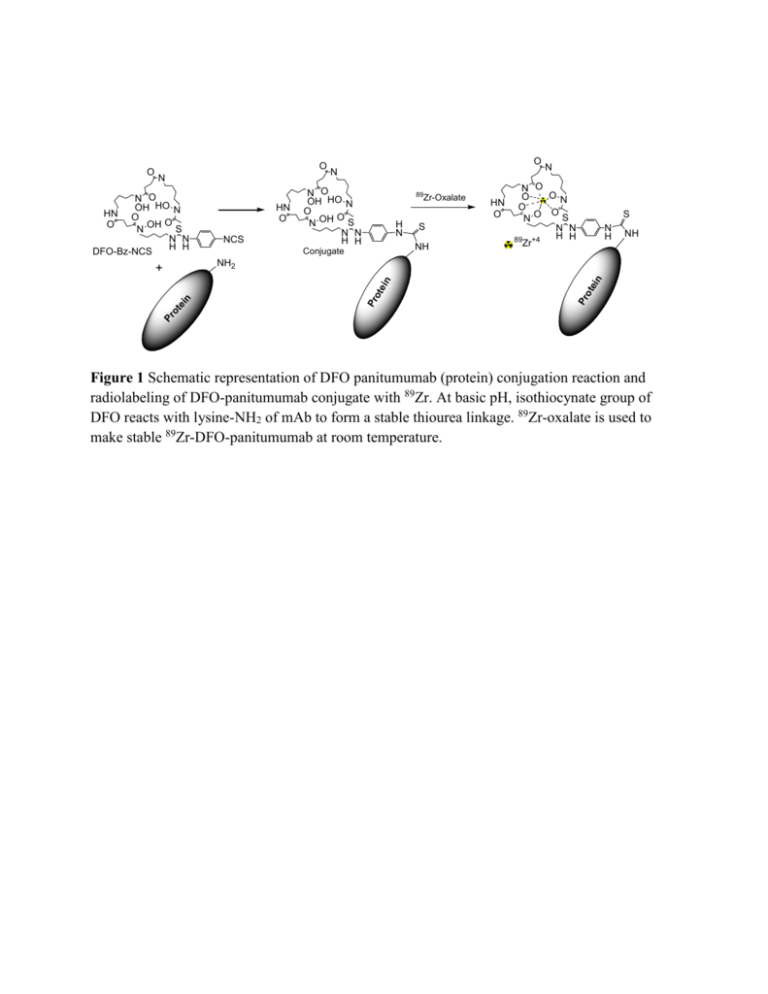
O N NCS 89 Zr-Oxalate H N S N H NH n NH2 Pr ot ei Pr ot ei n S NH Conjugate n + HN O N O OH HO N O O N OH S N N H H N N O ON O HN O O O S NO N N H H 89 +4 Zr tei N O OH HO N HN O O O N OH S N N H H DFO-Bz-NCS O N Pr o O Figure 1 Schematic representation of DFO panitumumab (protein) conjugation reaction and radiolabeling of DFO-panitumumab conjugate with 89Zr. At basic pH, isothiocynate group of DFO reacts with lysine-NH2 of mAb to form a stable thiourea linkage. 89Zr-oxalate is used to make stable 89Zr-DFO-panitumumab at room temperature. MDA-MB-468 MDA-MB-231 BT-474 HER-1 β-Actin Figure 2 A. Tumor uptake of 89Zr-panitumumab in various subcutaneous athymic nude female xenograft models. 10.18 ± 1.24 MBq of 89Zr-panitumumab were administered intravenously via tail-vein, and a 5 min CT scan followed by a 30 min static PET scan were performed at 96 h post-injection; B. Comparative EGFR expression level of BT-474, MDA-MB-231, and MDAMB-468 cell lines by western blot analysis. Cartesian molecular imaging software v5.0.2.30 and data is presented as EGFR/Actin ratio. The images are reprinted with the permission from Elsevier Inc. Bhattacharyya et al., Nucl. Med. Biol. 2013, 40, 451-457. mV Detector A Ch1:280nm 350 300 250 200 (a) 150 100 50 0 0.0 5.0 10.0 15.0 20.0 25.0 30.0 min mV Detector A Ch1:280nm 100 90 80 70 60 (b) 50 40 30 20 10 0 0.0 5.0 10.0 15.0 20.0 25.0 30.0 mi n Figure 3 HPLC chromatograms at 280 nm: (a) pure panitumumab; (b) DFO-panitumumab conjugate. 2 1.8 1.6 1.4 1.2 1 0.8 0.6 0.4 0.2 0 y = 8.2761x + 0.0065 R² = 0.9999 0 0.05 0.1 0.15 0.2 0.25 (a) mV Detector A Ch1:280nm 3 2 1 0 0.0 5.0 10.0 15.0 20.0 25.0 min (b) Figure 4 (a) Standard calibration curve using DFO-panitumumab conjugate as an authentic standard. X-axis represents the concentration in mg/mL (0.021 to 0.21mg/mL) and Y-axis represents the absorbance (peak area). (b) HPLC chromatogram for a 20 µL injection of a 0.021 mg/mL DFO-panitumumab conjugate showing conjugate’s UV sensitivity at 280 nm. Figure 5 Process flow of 89Zr-panitumumab synthesis for medical use. QC-1 and QC-2 are two in-process QC. Figure 6 HPLC chromatograms of 89Zr-panitumumab: (a) UV peak at 280 nm; (b) corresponding radiation peak. Early small shoulder of radiation peak could be due the 89Zr labeling to the trace amount of impurity (high molecular weight) present in the original protein.