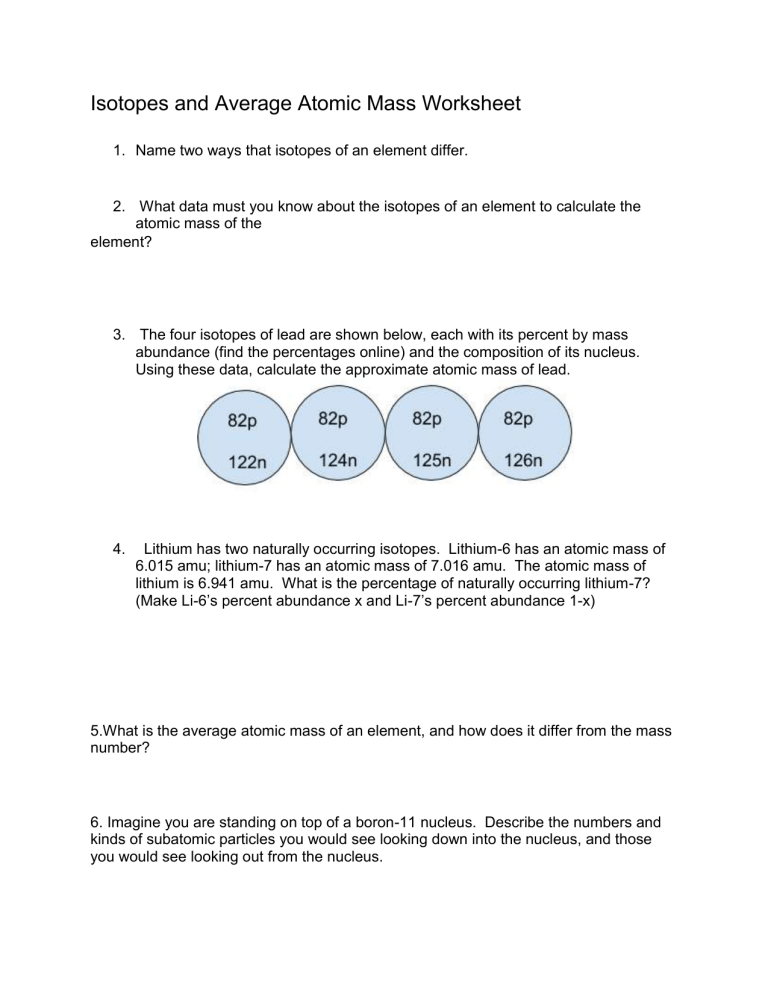
Isotopes and Average Atomic Mass Worksheet 1. Name two ways that isotopes of an element differ. 2. What data must you know about the isotopes of an element to calculate the atomic mass of the element? 3. The four isotopes of lead are shown below, each with its percent by mass abundance (find the percentages online) and the composition of its nucleus. Using these data, calculate the approximate atomic mass of lead. 4. Lithium has two naturally occurring isotopes. Lithium-6 has an atomic mass of 6.015 amu; lithium-7 has an atomic mass of 7.016 amu. The atomic mass of lithium is 6.941 amu. What is the percentage of naturally occurring lithium-7? (Make Li-6’s percent abundance x and Li-7’s percent abundance 1-x) 5.What is the average atomic mass of an element, and how does it differ from the mass number? 6. Imagine you are standing on top of a boron-11 nucleus. Describe the numbers and kinds of subatomic particles you would see looking down into the nucleus, and those you would see looking out from the nucleus. 7. The element boron, B, has an atomic mass of 10.81 amu according to the periodic table. However, no single atom of boron has a mass of exactly 10.81 amu. How can you explain this difference? 8. A certain element has two isotopes. One isotope, which has a percent abundance of 72.15% has a mass of 84.9118 amu. The other isotope has a mass of 86.9092 amu. a. Calculate the average atomic mass of this element to three decimal places. b. Identify the element. 9. Three isotopes of uranium occur in nature. If the relative atomic masses and abundances of each of these isotopes are as follows, calculate the average atomic mass of uranium to three decimal places.







