Acid-Base Titration Lab: NaOH Standardization & Unknown MW
advertisement

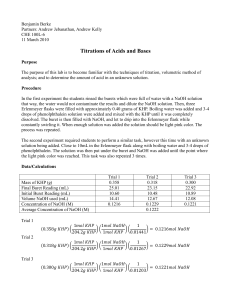
Lab #17 – Acid-Base Titration Name Background Titration is a lab procedure that takes advantage of a stoichiometric relationship between a known reagent and an unknown in order to determine the number of moles in the unknown sample. In acid-base titration we will use the one to one ratio between moles of H3O+ and OH- ions in the neutralization reaction. The tricky part in acid-base titration is making a solution of a known (aka, standard) whose concentration is known accurately. Some very common and inexpensive acids and bases make unsuitable standards. Hydrochloric acid is a solution of HCl gas dissolved in water; concentrated stock solutions of HCl have an unstable concentration due to the gradual loss of the gaseous solute. NaOH is also an unsuitable standard as the solid reacts gradually with CO2 and also absorbs water from the atmosphere. Both effects make accurate massing impossible under normal circumstances. We will work around this problem by starting with a less common, more expensive primary standard called potassium hydrogen phthalate, or KHP (MW=204.23 g/mol). This is a stable solid that can be weighed accurately. A carefully weighed sample will be reacted with a crudely prepared NaOH solution, which will then be our secondary standard. You will use the standardized NaOH solution to titrate against a carefully weighed sample of an unknown solid acid, allowing you to determine quite accurately the molecular weight of the unknown. Another concern with acid-base titration is that polyprotic acids can provide two or three moles of H3O+ ions. Absent knowledge of the unknown acids character - whether it is mono-, di-, or triprotic – we will be unable to determine molecular weight. Instead we can determine its equivalent mass, the mass of acid that is neutralized by one mole of OH- ions. Procedure Preparation and standardization of NaOH secondary standard: Do NOT spend a lot of time with careful measurement while preparing the NaOH standard. One partner should prepare the NaOH standard while the other prepares for the first titration as described below. Obtain about 7 mL of 6 M NaOH stock solution. Handle the NaOH with great care. Dilute this to about 400 mL in your 500 mL Erlenmeyer flask. Stopper the flask and swirl periodically over a 10 minute period to insure a uniform solution. While your partner is preparing the NaOH standard: Obtain a buret and an unknown acid sample. Record the unknown number and whether it is mono-, di-, or triprotic. Prepare the buret: Rinse the buret several times with copious quantities of tap water, being sure to allow several mL’s of water to rinse through the stopcock and tip. Next rinse the buret three times with 5-10 mL of distilled water. As a final step, the buret should be rinsed with about 5 mL of the NaOH standard, allowing all of the rinse to pass through the tip. Fill the buret with your NaOH standard, being sure not to fill past the top graduation on the buret. Briefly open the stopcock all the way to allow some solution to fill the buret tip. Check to make sure there are no bubbles trapped in the tip. In the prelab questions you calculated the amount of KHP that would react with about 30 mL of your NaOH standard. Preweigh this amount on the top-loading balance. On the analytic balance, weigh and record the mass of a clean, dry 125 mL Erlenmeyer flask. Transfer the KHP and weigh and record the mass of the flask with KHP. Dissolve the KHP in about 50 mL of water, and add 3 or 4 drops of phenolphthalein to the solution. After recording the initial volume of NaOH in the buret to the nearest .02 mL, begin dispensing the NaOH solution into the flask containing the KHP. One partner should swirl the flask continuously over a piece of white paper while the other controls the buret stopcock. Add NaOH rapidly in the beginning; as the pink color of phenolphthalein begins to persist, slow down. You will eventually add NaOH drop by drop, stopping after the one drop that causes a permanent color change. The endpoint, or equivalence point, is reached when the pink color of the phenolphthalein persists for at least 30 seconds. A quality endpoint is indicated by an extremely faint pink color. A dark color indicates the endpoint has been overshot. Do at least three trials. Determine the molecular weight of an unknown solid acid: Your unknown is labeled with an appropriate amount to use in titration. Using this amount, repeat the titration procedure you used with KHP. Do at least three trials. Data and Calculations Mass KHP (g) Moles KHP Initial Vol. (mL) Final Vol. (mL) Volume used (mL) [NaOH] Ave: Show a sample calculation of [NaOH]: UK # Mass UK (g) Monoprotic Moles UK Diprotic Initial Vol. (mL) Triprotic (circle one) Final Vol. (mL) Volume used (mL) MW of UK Ave: Show a sample calculation of the molecular weight: Prelab summary Procedural Tips: Rinse burets with tap water, DI water, then the titrant solution. Buret reminders: Stay between the lines, Fill the tip, Don’t forget to record Vi, Don't forget indicator. Safety: Use caution when handling 6 M NaOH. Rinse hands thoroughly after using. Burets are expensive and bulky. Use caution when manipulating burets. Disposal: All solutions can be rinsed down the sink. Prelab Questions 1. Your NaOH standard will be slapped together using about 7 mL of 6 M NaOH diluted to a total volume of approximately 400 mL. Calculate the approximate molarity of this standard. 2. How many grams of KHP will be needed to neutralize 30. mL of your NaOH standard? 3. A 0.6894 g sample of an unknown acid is neutralized by 42.62 mL of 0.1008 M NaOH solution. Find the molecular weight of the acid, assuming the acid is monoprotic. 4. What is the molecular weight of the acid in #3 above if the acid is diprotic? 5. What is the molecular weight of the acid in #3 above if the acid is triprotic?