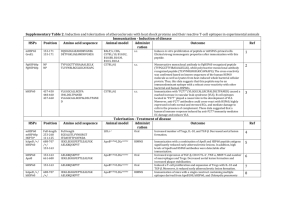
Supplementary Table 1. Amino acid sequence of heat shock
advertisement

Supplementary Table 1. Amino acid sequence of heat shock proteins and their reactive T-cell and B-cell epitopes from human samples Single T-cell epitopes HSPs Position Amino acid TCR Vβ Specimen Outcome sequence hHSP60, hHSP60p hHSP60, hHSP60p hHSP60, hHSP60p hHSP60, hHSP60p hHSP60, hHSP60p hHSP60, hHSP60p hHSP60, hHSP60p hHSP60, hHSP60p hHSP60, hHSP60p hHSP60, hHSP60p hHSP60, hHSP60p hHSP60, hHSP60p hHSP60, hHSP60p hHSP60, hHSP60p hHSP60, hHSP60p hHSP60, hHSP60p hHSP60, hHSP60p hHSP60, hHSP60p hHSP60, hHSP60p hHSP60, hHSP60p hHSP60, hHSP60p hHSP60, hHSP60p hHSP60, hHSP60p hHSP60, hHSP60p hHSP60, hHSP60p hHSP60, hHSP60p hHSP60, hHSP60p HSPs hHSP60, hHSP60p CpHSP60, CpHSP60p hHSP60, hHSP60p CpHSP60, CpHSP60p mHSP65, mHSP65p GroEL, GrpELp 1-15 6-20 11-25 31-45 91-105 96-110 136-150 141-155 201-215 206-220 226-240 261-275 321-335 331-345 466-480 491-505 506-520 246-260 266-280 281-295 326-340 416-430 421-435 436-450 471-485 476-490 551-565 Position 46-60 21-35 51-65 26-40 26-40 26-40 MLRLPTVFRQMRPVS TVFRQMRPVSRVLAP MRPVSRVLAPHLTRA KFGADARALMLQGVD KNIGAKLVQDVANNT KLVQDVANNTNEEAG NPVEIRRGVMLAVDA RRGVMLAVDAVIAEL VKDGKTLNDELEIIE TLNDELEIIEGMKFD PYFINTSKGQKCEFQ LEIANAHRKPLVIIA GGAVFGEEGLTLNLE TLNLEDVQPHDLGKV EIIKRTLKIPAMTIA VEKIMQSSSEVGYDA MAGDFVNMVEKGIID LSEKKISSIQSIVPA AHRKPLVIIAEDVDG EALSTLVLNRLKVGL GEEGLTLNLEDVQPH EKKDRVTDALNATRA VTDALNATRAAVEEG IVLGGGCALLRCIPA TLKIPAMTIAKNAGV AMTIAKNAGVEGSLI KEEKDPGMGAMGGMG Vβ11, Vβ9, Vβ5.2 Vβ14, Vβ8, Vβ14 Vβ1, Vβ8 Vβ20 Vβ4 Vβ21.3 Vβ5.2 Vβ12 Vβ17 Vβ3.1 Vβ4 Vβ5.1 Vβ2, Vβ12 Vβ18 Vβ22 Vβ11 Vβ7, Vβ6.7, Vβ16, Vβ11 NP NP NP NP NP NP NP NP NP NP LLADAVAVTMGPKGR KTLAEAVKVTLGPKG VAVTMGPKGRTVIIE AVKVTLGPKGRHVVI KVTLGPKGRNVVLEK AVKVTLGPKGRNVII Advanced atherosclerotic lesions and blood 4LL of Cp- patients+blood and 4 LL of Cp+ patients+blood. The submolecular specificity of HSP60-specific plaquederived T-cells was analyzed and both the self and crossreactive epitopes of that autoantigen were identified (see below). 1 Advanced and “healthy” atherosclerotic lesions and blood 7 EL and 8 LL patients. Blood from all EL, LL, and two HC groups consisting of 26 young and 14 old subjects. T-cells derived from LL displayed a more restricted T-cell receptor repertoire to hHSP60-derived peptides than those isolated from EL. This data supports the concept that hHSP60-reactive T-cells initiate atherosclerosis by recognition of atherogenic hHSP60 epitopes. 2 Cross-reactive T-cell epitopes TCR Vβ Specimen Amino acid sequence Vβ9 Vβ18 Ref Advanced atherosclerotic lesions and blood Outcome 4 LL of Cp- patients+blood and 4 LL of Cp+ patients+blood. All patients with positive serology and PCR detection of Chlamydia pneumoniae DNA had in their carotid plaques at least two populations of hHSP60 specific T-cells: one reactive only to self hHSP60, and the other reactive to both the self and the Chlamydia pneumoniae analog HSP60. Ref 1 1 hHSP60, hHSP60p CpHSP60, CpHSP60p hHSP60, hHSP60p mHSP65, mHSP65p CpHSP60, CpHSP60p GroEL, GrpELp hHSP60, hHSP60p CpHSP60, CpHSP60p hHSP60, hHSP60p mHSP65, mHSP65p CpHSP60, CpHSP60p GroEL, GrpELp hHSP60, hHSP60p mHSP65, mHSP65p CpHSP60, CpHSP60p GroEL, GrpELp hHSP60, hHSP60p CpHSP60, CpHSP60p hHSP60, hHSP60p CpHSP60, CpHSP60p hHSP60, hHSP60p CpHSP60, CpHSP60p hHSP60, hHSP60p CpHSP60, CpHSP60p hHSP60, hHSP60p CpHSP60, CpHSP60p hHSP60, hHSP60p CpHSP60, CpHSP60p hHSP60, hHSP60p CpHSP60, CpHSP60p hHSP60, hHSP60p CpHSP60, CpHSP60p hHSP60, hHSP60p CpHSP60, CpHSP60p hHSP60, hHSP60p CpHSP60, CpHSP60p hHSP60, hHSP60p CpHSP60, CpHSP60p PgHSP60/hHSP60 61-75 36-50 76-90 51-65 51-65 51-65 151-165 126-140 166-180 141-155 141-155 141-155 171-185 146-160 146-160 146-160 191-205 166-180 211-225 186-200 241-255 216-230 291-305 266-280 406-420 381-395 436-450 411-425 441-455 416-430 446-460 421-435 511-525 486-500 521-535 496-510 536-550 516-530 NP PgHSP60 NP TVIIEQSWGSPKVTK RHVVIDKSFGSPQVT DGVTVAKSIDLKDKY DGVSIAKEIELEDPY KDGVTVAKEIELEDK KDGVSVAKEIELEDK VIAELKKQSKPVTTP VVVDELKKISKPVQH EEIAQVATISANGDK QIAATAAISAGDQSI HKEIAQVATISANND SEEVAQVGTISANGD VATISANGDKEIGNI TAAISAGDQSIGDLI QVATISANNDSEIGN QVATISANGDKQVGL KKVGRKGVITVKDGK MEKVGKNGSITVEEA LEIIEGMKFDRGYIS VLDVVEGMNFNRGYL DAYVLLSEKKISSIQ EDALILIYDKKISGI LKVGLQVVAVKAPGF RLRAGFRVCAVKAPG VGGTSDVEVNEKKDR VGAATEIEMKEKKDR IVLGGGCALLRCIPA ILPGGGTALVRCIPT GCALLRCIPALDSLT GTALVRCIPTLEAFL RCIPALDSLTPANED RCIPTLEAFLPMLAN VNMVEKGIIDPTKVV AYTDMIDAGILDPTK PTKVVRTALLDAAGV LDPTKVTRSALESAA ASLLTTAEVVVTEIP LLTTEALIADIPEEK TVPGGGTTYIRAIAALEGLK TLVVNRLRGSLKICAVKAPG Vβ5.1 Vβ17 Vβ14 Vβ11 Vβ12 Vβ5.2 Vβ4 Vβ13.1 Vβ8 Vβ14 Vβ9 Vβ1 Vβ13.2 Vβ22 Vβ9 Vβ11 NP Advanced atherosclerotic lesions, gingival tissue, and blood 20 LL and periodontitis, 20 with periodontitis, and 20 HC. Thirty per cent of periodontitis patients and 100% of atherosclerosis patients reacted positively to the crossreactive peptide (TLVVNRLRGSLKICAVKAPG) from both Pg and hHSP60. The peptide for a specific T-cell line 3 2 demonstrated the phenotype characteristic of helper Tcells (CD4+) but did not express CD25 or FOXP3 and IL-10 was elevated. Thus, this cross-reactive peptide is an immunoreactive epitope in the periodontis-atherosclerosis axis. HSPs Position Amino acid sequence hHSP60 1-573 (full-length) NP hHSP60 1-573 (full-length) NP hHSP60 1-573 (full-length) NP hHSP60, PgHSP60 NP NP hHSP60, GroEl, PgHSP60 NP hHSP60, CpHSP60 NP TCR Vβ without amino acid sequence TCR Vβ Specimen Outcome Ref Asym: Vβ1, Vβ2, Vβ3, Vβ7, Vβ9, Vβ11, Bβ16, Vβ20 Sym: Vβ3, Vβ6.2, Vβ7, Vβ9, Vβ11, Vβ13, Vβ18, Vβ20, Vβ21, Vβ24 AS: Vβ2, Vβ3, Vβ4, Vβ5.1, Vβ6.1, Vβ6.2, Vβ8, Vβ11, Bβ12, Vβ14, Vβ17, Vβ18, Vβ21, Vβ24 Vβ2, Vβ5.2, Vβ6, Vβ8, Vβ9, Vβ12, Vβ13.1, Vβ16, Vβ18, Vβ19, Vβ20 Advanced atherosclerotic lesions and blood 10 LL, 5 with asymptomatic and 5 with symptomatic stenosis. Blood from these patients was obtained twice, on the date of endarterectomy and 2 weeks after surgery. 6 additional LL and blood from atherosclerotic patients were used. Lesion derived T-cells displayed an oligoclonallyrestricted repertoire, in contrast to the polyclonal pattern of PBMC. This indicates that HSP60 may be a major antigenic candidate and that oligoclonal T-cell expansion takes place in advanced human atherosclerotic lesions. 4 Advanced atherosclerotic lesions, gingival tissue, and blood 5 NP Vβ5.2-3, Vβ13.1/13.3 Advanced atherosclerotic lesions and blood NP Both CD4+ and CD8+ Tcells with a majority bearing the αβ TCR rather than γδ TCR. Advanced atherosclerotic lesions and blood 15 LL and periodontitis, 16 with periodontitis, and 10 HC. Antibody levels to both hHSP60 and PgHSP60 were highest in atherosclerosis patients, followed by periodontitis patients and healthy subjects. Clonal analysis of the T-cells clearly demonstrated the presence of not only hHSP60 but also PgHSP60-reactive T-cell populations in the peripheral circulation of atherosclerosis patients. These HSP60reactive T-cells were present in atherosclerotic lesions in some patients. 25 patients with LL and blood from 22 of these patients. A cross-reactivity of several T-cell lines was demonstrated. The cytokine profiles of the arterial T-cell lines specific for hHSP60, GroEL, and PgHSP60 displayed a Th2 phenotype predominance in CD4+ cells. A higher proportion of CD4 cells was positive for IFN-IP10 and RANTES, with low percentages of cells positive for MCP-1 and MCP-1α, whereas a high percentage of CD8+ cells expressed all four chemokines. Finally, there was overexpression of the TCR Vβ5.2-3 family in all lines. 32 patients with LL and blood. Antigen responsiveness of T-cell lines showed that those derived using Chlamydia organisms were more likely to respond to Chlamydia than those isolated using other stimuli. Chlamydia-specific Tcell lines were shown to respond to OMP2 and/or hHSP60, those recognizing CpHSP60 did not cross-react with hHSP60, but hHSP60-responsive lines were also observed. 6 7 3 Thus, atherosclerotic plaque tissue contains a variety of memory T-cells, and amongst these are cells capable of recognizing Chlamydia antigens. HSPs hHSP60p mHSP65p CpHSP60p GroELp hHSP60p mHSP65p CpHSP60p GroELp hHSP60p mHSP65p CpHSP60p GroELp hHSP60p mHSP65p CpHSP60p GroELp hHSP60p mHSP65p CpHSP60p GroELp hHSP60p mHSP65p CpHSP60p GroELp hHSP60p mHSP65p CpHSP60p GroELp hHSP60p mHSP65p CpHSP60p GroELp hHSP60p mHSP65p hHSP60p mHSP65p hHSP60p Position 86-98 61-73 61-73 61-73 116-128 91-103 91-103 91-103 131-148 91-103 91-103 91-103 186-203 161-178 161-178 161-178 241-258 216-233 216-233 216-233 261-273 236-248 236-248 236-248 461-478 436-453 436-453 436-453 516-528 491-503 491-503 491-503 52-61 26-35 57-66 31-40 62-71 Cross-reactive B-cell epitopes Amino acid sequence Specimen LKDKYKNIGAKLV LEDPYEKIGAELV LADKHENMGAQMV LEDKFENMGAQMV ATVLARSIAKEGF ATVLAQALVREGL ATVLAEAIYTEGL ATVLAQAIITEGL ISKGANPVEIRRGVMLAV VAAGANPLGLKRGIEKAV VTAGANPMDLKRGIDKAV VAAGMNPMDLKRGIDKAV ISDAMKKVGRKGVITVKD IAEAMDKVGNEGVITVEE IAEAMEKVGKNGSITVEE IAEAMDKVGKEGVITVED DAYVLLSEKKISSIQSIV DPYILLVSSKVSTVKDLL DALVLIYDKKISGIKDFL SPFILLADKKISNIREML LEIANAHRKPLVI LEKVIGAAKPLLI LQQVAESGRPLLI LEAVAKAGKPLLI QKIGIEIIKRTLKIPAMT EATGANIVKVALEAPLKQ EQIGARIVLKALSAPLKQ QNVGIKVALRAMEAPLRQ KGIIDPTKVVRTA AGVADPVKVTRSA AGILDPAKVTRSA MGILDPTKVTRSA AVTMGPKGRT KVTLGPKGRN PKGRTVIIEQ PKGRNVVLEK VIIEQSWGSP Outcome Ref Blood Sera from 5 subjects with ≥1:1280 anti-HSP antibodies and sonographically proven atherosclerosis. Antibodies to microbial HSP60/65 recognize specific epitopes on hHSP60. These cross-reactive epitopes were shown to serve as autoimmune targets in incipient atherosclerosis. 8 Blood Purified Ig preparation from pooled plasma of >6000 healthy blood donors and a second set of samples were collected from 12 healthy blood donors. Three epitopes were “specific” for hHSP60 and three different epitopes were “specific” for mHSP65. In addition, eight epitopes 9 4 mHSP65p hHSP60p hHSP60p hHSP60p mHSP65p hHSP60p hHSP60p mHSP65p hHSP60p mHSP65p mHSP65p hHSP60p mHSP65p hHSP60p mHSP65p mHSP65p mHSP65p hHSP60p mHSP65p hHSP60p mHSP65p hHSP60p mHSP65p 36-45 67-76 72-81 117-126 91-100 132-141 137-146 111-120 218-227 191-200 276-285 394-403 366-375 441-450 413-422 502-511 507-516 97-109 VVLEKKWGAP SWGSPKVTKD KVTKDGVTVA TVLARSIAKE TVLAQALVRE SKGANPVEIR PVEIRRGVML PLGLKRGIEK KFDRGYISPY RFDKGYISGY PGFGDRRKAM LAKLSDGVAV LAKLAGGVAV GCALLRCIPA GVTLLQAAPT NAASIAGLFL AGLFLTTEAV ALVREGLRNVAAG 179-187 NTFGLQLEL 504-512 AASIAGLFL mHSP60 hHSP60p mHSP65p mHSP60 mHSP65p hHSP60 PgHSP60 hHSP60 PgHSP60 hHSP60 PgHSP60 hHSP60 PgHSP60 hHSP60p PgHSP60p hHSP60p PgHSP60p HpHSP60p NP 40, 43-45, 386 40, 43-46 NP 178-181, 183 71-80 73-82 140-149 142-151 260-269 262-271 277-286 279-288 340-349 342-351 365-374 367-376 141-160 CIGSPSYNC QGSPV KGAPT CSFHYQNRC NTFGQ VQDVANNTNE VKEVASKTND EEIAQVATIS QKIEHVAKIS LVLNRLKVGL LVVNRLRGSL PGFGDNRKNQ PGFGDRRKAM QIEKRIQEII GIASRITQIK NERLAKLSDG QERLAKLAGG EEITQVATISANSDHNIGKL were cross-reactive in nature with a homology of 40-70%. The presence of these “specific” epitopes may explain the differences in epitope structure between hHSP60 and mHSP65 observed in patients with cardiovascular disease. Blood Blood Advanced atherosclerotic lesions and blood Blood High-titer sera from 10 subjects with ≥1:1280 anti-hHSP60 and anti-hHSP60 antibodies and sonographically proven atherosclerosis. Anti-HSP60/65 antibodies from subjects with atherosclerotic lesions react specifically with three short, linear epitopes present in HSP60/65, which may be involved as autoantigens in the pathogenesis of atherosclerosis. Sera from 5 subjects with ≥1:1280 anti-HSP antibodies and sonographically proven atherosclerosis. Two atherosclerosis-associated conformational HSP60 epitopes were defined by the use of phage display and structural alignment. 6 LL Pg+ patients and 6 HC. Blood from both patients and HC. Six cross-reactive B-cell epitopes of PgHSP60 and hHSP60 were defined in atherosclerosis patients with a concomitant periodontal disease. 10 Blood from 250 CVD patients and 293 non-CVD patients. IgG antibodies against this particular amino acid sequence 13 11 12 5 hHSP60 HSPs PgHSP60 409-424 TSDVEVNEKKERVTEA Cross-reactive T-cell and B-cell epitopes Amino acid sequence Specimen Position 12-21 73-82 162-171 262-271 493-502 Blood RDLLKKGVDA VKEVASKTND IAEAMRKVKK LVVNRLRGSL VIDPAKVTRV Advanced atherosclerotic lesions and blood of HpHSP60p and anti-hHSP60 predominantly appeared in CVD patients compared to controls. Furthermore, neither titer of HpHSP60p nor anti-hHSP60 antibodies was correlated with the levels of high sensitive C-reactive protein (hsCRP). Thus, IgG anti-HpHSP60p antibodies cross-reacting with hHSP60 might be independent diagnostic markers relevant to CVD. Blood from 35 ACS patients (12 with UA and 23 with MI) and 20 HC. Levels of specific serum antibodies against hHSP60 were significantly elevated in ACS patients. One immunodominant region was revealed corresponding to the hHSP60(409-424) peptide. None of the seven hHSP60 sequences tested corresponded to the mHSP65 epitopes (97-109, 179-187, and 504-512). 14 Outcome Ref 6 LL Pg+ patients and 6 HC. Blood from both patients and HC. Five immunodominant T-cell and B-cell epitopes of PgHSP60 was defined in atherosclerosis patients with a periodontal disease. This indicates that PgHSP60 might be involved in the immunoregulatory process of atherosclerosis. 12 hHSP60=human heat shock protein (hHSP) 60 (573 amino acid long), hHSP60p=hHSP60 peptide, mHSP65=Mycobacterium bovis heat shock protein (mHSP) 65 (540 amino acid long), mHSP65p=mHSP65 protein, CpHSP60=Chlamydia pneumonia HSP60 (544 amino acid long), PgHSP60=Porphyromonas gingivalis HSP60, GroEL=GroELfrom Escherichia coli, HpHSP60=Helicobacter pylori HSP60, GroELp=GroEL peptide, LL=Late lesion, EL=Early lesion, Asym=Asymptomatic, Sym= Symptomatic, AS=Atherosclerosis, CVD=cardiovascular disease, ACS=acute coronary syndrome, UA=Unstable angina, MI=myocardial infarction, NP=not performed, HC=healthy control, IFN-IP10=Interferon-inducible protein 10, MCP-1=monocyte chemoattractant protein 1, MIP-1α=macrophage inflammatory protein 1α,*HC=healthy control with sonographically proven carotid atherosclerosis References 1. Benagiano, M. et al. Human 60-kDa heat shock protein is a target autoantigen of T cells derived from atherosclerotic plaques. J Immunol 174, 6509-17 (2005). 2. Almanzar, G. et al. Autoreactive HSP60 epitope-specific T-cells in early human atherosclerotic lesions. J Autoimmun 39, 441-50 (2012). 3. Choi, J., Lee, S.Y., Kim, K. & Choi, B.K. Identification of immunoreactive epitopes of the Porphyromonas gingivalis heat shock protein in periodontitis and atherosclerosis. J Periodontal Res 46, 240-5 (2011). 6 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. Rossmann, A. et al. T-cells from advanced atherosclerotic lesions recognize hHSP60 and have a restricted T-cell receptor repertoire. Exp Gerontol 43, 229-37 (2008). Yamazaki, K. et al. T-cell clonality to Porphyromonas gingivalis and human heat shock protein 60s in patients with atherosclerosis and periodontitis. Oral Microbiol Immunol 19, 160-7 (2004). Ford, P. et al. Characterization of heat shock protein-specific T cells in atherosclerosis. Clin Diagn Lab Immunol 12, 259-67 (2005). Curry, A.J., Portig, I., Goodall, J.C., Kirkpatrick, P.J. & Gaston, J.S. T lymphocyte lines isolated from atheromatous plaque contain cells capable of responding to Chlamydia antigens. Clin Exp Immunol 121, 261-9 (2000). Perschinka, H. et al. Cross-reactive B-cell epitopes of microbial and human heat shock protein 60/65 in atherosclerosis. Arterioscler Thromb Vasc Biol 23, 1060-5 (2003). Uray, K., Hudecz, F., Fust, G. & Prohaszka, Z. Comparative analysis of linear antibody epitopes on human and mycobacterial 60kDa heat shock proteins using samples of healthy blood donors. Int Immunol 15, 1229-36 (2003). Metzler, B. et al. Epitope specificity of anti-heat shock protein 65/60 serum antibodies in atherosclerosis. Arterioscler Thromb Vasc Biol 17, 536-41 (1997). Perschinka, H. et al. Identification of atherosclerosis-associated conformational heat shock protein 60 epitopes by phage display and structural alignment. Atherosclerosis 194, 79-87 (2007). Choi, J.I. et al. Epitope mapping of Porphyromonas gingivalis heat-shock protein and human heat-shock protein in human atherosclerosis. J Dent Res 83, 936-40 (2004). Okada, T. et al. Antibodies against heat shock protein 60 derived from Helicobacter pylori: diagnostic implications in cardiovascular disease. J Autoimmun 29, 106-15 (2007). Wysocki, J. et al. Human heat shock protein 60 (409-424) fragment is recognized by serum antibodies of patients with acute coronary syndromes. Cardiovasc Pathol 11, 238-43 (2002). 7