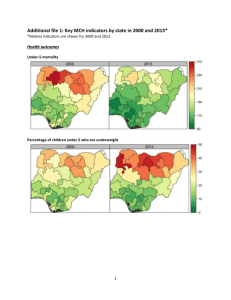
Supplementary Table 2. Induction and tolerization of atherosclerosis
advertisement

Supplementary Table 2. Induction and tolerization of atherosclerosis with heat shock proteins and their reactive T-cell epitopes in experimental animals Immunization - Induction of disease HSPs Position Amino acid sequence Animal model Administ Outcome Ref ration mHSP65 GroEL 153-171 153-171 DQSIGDLIAEAMDKVGNEG DETVGKLIAEAMDKVGKEG PgHSP60p PgHSP60p NP NP TVPGGGTTYIRAIAALEGLK TLVVNRLRGSLKICAVKAPG hHSP60 437-450 448-460 437-460 VLGGGCALLRCIPA IPALDSLTPANED VLGGGCALLRCIPALDSLTPANE D s.c. Induces in vitro proliferation of peptide or mHSP65-primed cells. Elicited strong immunogenic properties after immunization with this peptide. 1 s.c. 2 C57BL/6J s.c. Monoreactive monoclonal antibody to PgHSP60 recognized peptide (TVPGGGTTYIRAIAALEGLK), while polyreactive monoclonal antibody recognized peptide (TLVVNRLRGSLKICAVKAPG). The cross-reactivity was confirmed based on known sequences of the human HSP60 molecule as well as lysates from heat-induced whole bacterial cellular protein. Thus, this data suggests that this peptide may be an immunodominant epitope with a robust cross-reactivity with other bacterial and human HSP60s. Immunization with “P277” (VLGGGCALLRCIPALDSLTPANED) caused a marked increase in vascular leak syndrome (VLS). B-cell epitopes located in “P277” played a causal role in the development of VLS. Moreover, anti-P277 antibodies could cross-react with HSP60, highly expressed in both normal and stressed ECs, and mediate damage to cells in the presence of complement. These data suggested that a humoral immune response induced by anti-P277 immunity mediates EC damage and induces VLS. Tolerization - Treatment of disease Animal model Administ ration 3 HSPs Position mHSP60 mHSP60p HSP70* hApoB /+/ hHSP60 Full-length 253-268 111-125 688-707 /+/ 153-163 Full-length EGEALSTLVVNKIRGT ITDAVITTPAYFNDA IEIGLEGKGFEPTLEALFGK AELKKQSKPVT LDLr-/- Oral Increased number of Tregs, IL-10, and TGF-β. Decreased aortal lesion formation. 4 ApoBtm25gyLDLrtm1Her RIMMS 5 hHSP60 ApoB 153-163 661-680 AELKKQSKPVT IEIGLEGKGFEPTLEALFGK ApoBtm25gyLDLrtm1Her Oral hHSP60 153-163 AELKKQSKPVT ApoBtm25gyLDLrtm1Her Oral IEIGLEGKGFEPTLEALFGK AELKKQSKPVT ApoBtm25gyLDLrtm1Her RIMMS Immunization with a combination of ApoB and HSP60 peptide antigens significantly reduced early atherosclerotic lesions. In addition, high levels of ApoB and HSP60 antibodies were detectable after immunization. Increased expression of TGF-β, CD4+CTL-4+, TNF-α, MMP-9 and number of macrophages and Tregs. Decreased aortal lesion formation and increased plaque stabilization. Induced a T-cell proliferation and expansion of Tregs with IL-10 and TGF-β. Moreover, it reduced early atherosclerotic lesion formation. Immunization of mice with a single construct containing multiple epitopes derived from ApoB100, hHSP60, and Chlamydia pneumonia hApoB /+/ hHSP60 688-707 /+/ Amino acid sequence BALP/c, CBA, C57BL/10, B10.D2, B10.BR, B10.M, B10.S, B10.A C57BL/6J Outcome Ref 6 7 8 /+/ hHSP60 /+/ MOMP /+/ Cp mHSP65 mHSP65 mHSP65 mHSP65 153-163 /+/ 303-312 /+/ 67-74 /+/ 283-291 91-105 106-125 111-125 312-326 PGFGDNRKNQ GDYVFDRI QAVANGGAI ATVLAQALVREGLRN VAAGANPLGLKRGIEKAVEK NPLGLKRGIEKAVEK LSLLGKARKVVVTKD was more effective in reducing early atherosclerotic lesions through the induction of a specific Treg-cell response than was the construct containing either mono- or bi-epitopes. New Zealand white rabbits + C57BL/6J + LDLr-/- s.c. Atherosclerosis was significantly enhanced in HCD fed immunized rabbits. Immunized mice had increased aortic endothelial injury. Although western blot demonstrated that specific antibodies against mHSP65(91-105) can cross-react with recombinant hHSP60, specific antibodies against mHSP65(91-105) had no direct effects on HUVECs in vitro, even when the HUVECs were heat shocked. Adoptive transfer of mHSP65(91-105) specific splenic cells can accelerate atherosclerosis in LDLr-/- mice. 9 s.c.=subcutaneous, RIMMS=The repetitive immunization multiple sites strategy, Cp=Chlamydia pneumonia, PgHSP60=Porphyromonas gingivalis HSP60, HCD=highcholesterol diet, NP=Not performed. *=Sequence was based on a partially conserved human, rat, and mouse sequence of the HSP70 molecule. References 1. Brett, S.J. et al. Differential pattern of T cell recognition of the 65-kDa mycobacterial antigen following immunization with the whole protein or peptides. Eur J Immunol 19, 1303-10 (1989). 2. Choi, J., Lee, S.Y., Kim, K., Choi, B.K. & Kim, M.J. Identification of mono- or poly-specific monoclonal antibody to Porphyromonas gingivalis heat-shock protein 60. J Periodontal Implant Sci 41, 54-9 (2011). 3. Yong, L. et al. Immunization with P277 induces vascular leak syndrome in C57BL/6 mice via endothelial damage. Autoimmunity 43, 654-63 (2010). 4. van Puijvelde, G.H. et al. Induction of oral tolerance to HSP60 or an HSP60-peptide activates T cell regulation and reduces atherosclerosis. Arterioscler Thromb Vasc Biol 27, 2677-83 (2007). 5. Lu, X. et al. Immunization with a combination of ApoB and HSP60 epitopes significantly reduces early atherosclerotic lesion in Apobtm2SgyLdlrtm1Her/J mice. Atherosclerosis 212, 472-80 (2010). 6. Mundkur, L. et al. Mucosal tolerance to a combination of ApoB and HSP60 peptides controls plaque progression and stabilizes vulnerable plaque in Apob(tm2Sgy)Ldlr(tm1Her)/J mice. PLoS One 8, e58364 (2013). 7. Mundkur, L.A. et al. Activation of inflammatory cells and cytokines by peptide epitopes in vitro: a simple in-vitro screening assay for prioritizing them for in-vivo studies. Inflamm Res 62, 471-81 (2013). 8. 9. Lu, X. et al. Impact of multiple antigenic epitopes from ApoB100, hHSP60 and Chlamydophila pneumoniae on atherosclerotic lesion development in Apob(tm2Sgy)Ldlr(tm1Her)J mice. Atherosclerosis 225, 56-68 (2012). Zhang, Y. et al. A novel atherogenic epitope from Mycobacterium tuberculosis heat shock protein 65 enhances atherosclerosis in rabbit and LDL receptor-deficient mice. Heart Vessels 27, 411-8 (2012).