Click
advertisement

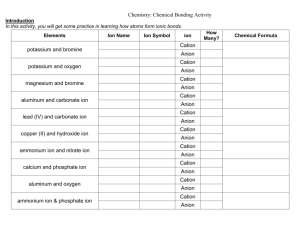
MIDDLE EAST TECHNICAL UNIVERSITY NORTHERN CYPRUS CAMPUS Chemical Nomenclature Step 1 Identify the compound type as one in the list given below. Step 2 Name the compound according to the guidelines given for that compound type (below). Compound Types I. IONIC Compound A. consists of monoatomic ions only (metal and nonmetal) B. includes polyatomic ion(s) II. MOLECULAR Compound (consists of nonmetals only) III: Acids and Bases IV. Hydrates Rules for Naming by Type IA. Ionic Compound consisting of monoatomic ions 1) First list cation name (cation name is the element name). IF the cation is a transition metal cation, follow cation name by Roman numeral, in parentheses that gives the cation’s charge. 2) Follow cation name with anion name. (Anion name is element stem name plus -ide.) Examples: Mg3N2 magnesium nitride Fe2O3 iron (III) oxide IB. Ionic Compound including polyatomic ion(s) 1) First list cation name (cation name is the element name). IF the cation is a transition metal cation, follow cation name by Roman numeral, in parentheses that gives the cation’s charge. [IF the cation is NH4+ then the cation name is ammonium ion.] 2) Follow cation name with anion name (or possibly monoatomic anion name – if NH4+ is the cation). Examples: FeSO4 iron (II) sulphate KMnO4 potassium permanganate NOTE: Ionic compounds DO NOT use the prefixes di-, tri-, etc. II. Molecular Compounds 1) Find the element that appears first in this list: B Si C Sb As P N H Te Se S I Br Cl O F 2) List this element’s name. (You can just list first the element that appears first in the formula). 3) Follow this name with the stem name of the other element plus -ide. 4) Add the prefix to each of these names that identifies the number of atoms of the element in the formula. (Typically you do not need to list mono-.) Examples: P4O6 tetraphosphorus hexoxide CO2 carbon dioxide NOTE: If a prefix ends in a vowel and the element starts with a vowel, drop the vowel at the end of the prefix. (hexa plus oxide becomes hexoxide) III. Acids and Bases Naming acids may look arbitrary and ambiguous. Simple acids are easily named. For example, HCl in water is named as hydrochloric acid. Oxoacids contain hydrogen, oxygen and another element that acts the central atom. Here are some examples: H2CO3 Carbonic acid HClO3 Chloric acid HNO3 Nitric acid H3PO4 Phosphoric acid H2SO4 Sulphuric acid Bases are easier to name. Almost all bases have a monatomic cation. The anion usually is the hydroxide ion. So rules for ionic compounds apply to them equally. One exception is ammonia, NH3. When dissolved in water it yields NH 4 and OH ions. III. Hydrates 1) Write correct name of compound without water molecules. 2) Follow compound name by prefix that identifies the number of water molecules bound to the compound. 3) Follow the prefix with hydrate. Example: CuSO4 • 5H2O copper (II) sulphate pentahydrate 2