Names of Ionic Compounds
advertisement
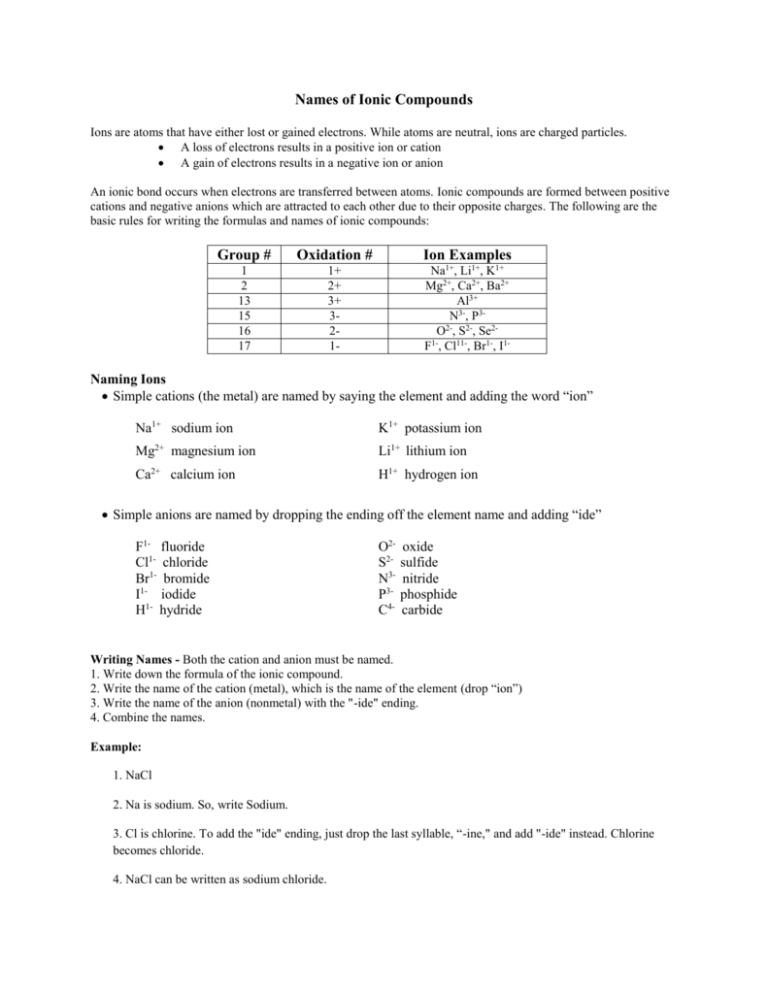
Names of Ionic Compounds Ions are atoms that have either lost or gained electrons. While atoms are neutral, ions are charged particles. A loss of electrons results in a positive ion or cation A gain of electrons results in a negative ion or anion An ionic bond occurs when electrons are transferred between atoms. Ionic compounds are formed between positive cations and negative anions which are attracted to each other due to their opposite charges. The following are the basic rules for writing the formulas and names of ionic compounds: Group # Oxidation # Ion Examples 1 2 13 15 16 17 1+ 2+ 3+ 321- Na1+, Li1+, K1+ Mg2+, Ca2+, Ba2+ Al3+ N3-, P32O , S2-, Se21F , Cl11-, Br1-, I1- Naming Ions Simple cations (the metal) are named by saying the element and adding the word “ion” Na1+ sodium ion K1+ potassium ion Mg2+ magnesium ion Li1+ lithium ion Ca2+ calcium ion H1+ hydrogen ion Simple anions are named by dropping the ending off the element name and adding “ide” F1- fluoride Cl1- chloride Br1- bromide I1- iodide H1- hydride O2S2N3P3C4- oxide sulfide nitride phosphide carbide Writing Names - Both the cation and anion must be named. 1. Write down the formula of the ionic compound. 2. Write the name of the cation (metal), which is the name of the element (drop “ion”) 3. Write the name of the anion (nonmetal) with the "-ide" ending. 4. Combine the names. Example: 1. NaCl 2. Na is sodium. So, write Sodium. 3. Cl is chlorine. To add the "ide" ending, just drop the last syllable, “-ine," and add "-ide" instead. Chlorine becomes chloride. 4. NaCl can be written as sodium chloride. Writing Formulas 1. Determine the formulas and charges on the cation and anion involved in the compound. 2. Combine the ions in a ratio that results in the formation of a neutral ionic compound. In other words, the total charge of all the positive cations must equal the total charge of all the negative anions in the compound. The numbers of each element present in the compound are shown as subscripts after the element symbol. Example: Write the formula and name for the compound formed between calcium and fluorine. Ca (metal) forms a 2+ cation Ca2+ the calcium cation. F (non-metal) forms a 1- anion F1- the fluoride anion. To obtain a neutral compound, 1 Ca2+ is needed for every 2 F1The formula of the compound is CaF2 The name of the compound is Calcium Fluoride Shortcut for Formula Determination: Use the following method when asked to determine the formula of an ionic compound: 1. Write the two ions with their charges (metal first). 2. Ignoring the + or – charges, “crisscross” the numbers and make them subscripts. 3. Then, rewrite the formula, dropping the charges. (See Examples Below) Example 1: Write the formula for calcium chloride: 1. Write the two ions with their charges (metal first). Ca2+ Cl12. Ignoring the + or – charges, “crisscross” the numbers and make them subscripts: Ca2+ Cl13. Then, rewrite the formula, dropping the charges. In this case, the formula is: CaCl2. Example 2: Write the formula for magnesium oxide: 1. Write the two ions with their charges (metal first). Mg2+ O22. Ignoring the + or – charges, “crisscross” the numbers and make them subscripts: Mg2+ O23. Then, rewrite the formula, dropping the charges. The rewritten formula is: Mg2O2. Note: Since the subscripts for the anion and cation are the same, the formula reduces to Mg1O1. Therefore, the correct formula is written as: MgO.











