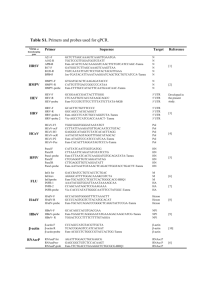
Supplemental Table 1. Probe information for TAPBP polymorphisms
advertisement

Supplemental Table 1. Probe information for TAPBP polymorphisms Loci assay ID rs3106189 C___2479333_10 rs2239841 Forward GGCACCTTCACCTAACCAGA Reverse CCTCTAGTTCTTGGGCGATG VIC AGAGGAGGCTTAATGGCTGAG FAM AGAGGAGGCTTCATGGCTGAGG rs3106190 C___2479337_20 rs2071888 C__11407883_1_ rs1059288 C___2479349_10 Supplemental table 2. Allele frequency and Hardy-Weinberg equilibrium in study subjects Loci Position rs3106189 5'UTR rs2239841 rs3106190 rs2071888 rs1059288 Amino acid 5'UTR Intron3 Exon4 3'UTR Genotype change Thr260Arg G AG A N 613 530 93 1236 C AC A N 784 416 40 1240 G CG C N 620 532 92 1244 C CG G N 551 562 128 1241 T CT C N 553 562 127 1242 MAF Heterozygosity HWE 0.290 0.412 0.139 0.200 0.320 0.088 0.288 0.410 0.127 0.330 0.442 0.383 0.329 0.441 0.366 Supplemental table 3. Genotype and haplotype distributions of TAPBP gene between the subjects with nasal polyps and those without nasal polyps. Nasal Polyp Loci position Y rs3106189 5'UTR rs3106190 5'UTR Intron3 139(49.8%) 352(50.1%) AG 122(43.7%) 297(42.3%) C Exon4 3'UTR _ 180(65.2%) 450(63.7%) 234(33.1%) A 8(2.9%) 22(3.1%) G 141(50.0%) 353(50.2%) CG 123(43.6%) 297(42.3%) 18(6.4%) Recessive 125(44.6%) 316(45.0%) CG 132(47.1%) 308(43.8%) 23(8.2%) 127(45.0%) 316(45.1%) CT 132(46.8%) 307(43.8%) -/- p OR((5% CI) p OR((5% CI) p 0.92(0.73-1.15) 0.45 0.95(0.71-1.27) 0.73 0.72(0.40-1.28) 0.26 0.90(0.69-1.17) 0.42 0.89(0.66-1.21) 0.45 0.81(0.35-1.91) 0.64 0.92(0.73-1.16) 0.46 0.95(0.71-1.27) 0.73 0.72(0.41-1.29) 0.27 0.85(0.68-1.06) 0.16 0.91(0.68-1.22) 0.53 0.59(0.36-0.99) 0.04 0.85(0.68-1.06) 0.14 0.90(0.67-1.20) 0.48 0.59(0.36-0.99) 0.04 1.00(0.71-1.41) 0.99 1.02(0.70-1.49) 0.91 0.77(0.19-3.23) 0.73 79(11.2%) T 23(8.2%) OR((5% CI) 53(7.5%) C C ht3 Dominant 54(7.7%) 88(31.9%) G rs1059288 18(6.5%) AC C rs2071888 N G A rs2239841 Codominant genotype 78(11.1%) 230(81.9%) 581(82.9%) ht3/- 48(17.1%) 113(16.1%) ht3/ht3 3(1.1%) 7(1.0%) MAF: minor allele frequency, OR: odds ratio, CI: confidence interval. P-value is adjusted for age, sex, smoking and atopy as co-variables. Supplemental table 4. Comparisons of haplotype distributions of TAPBP between the subjects with AERD and those with ATA MAF Global analysis Haplotype Referent analysis p- AERD ATA OR pOR value value ht1 0.616 0.685 1 ht2 0.219 0.194 1.24(0.97-1.59) 0.09 ht3 0.104 0.085 1.19(1.00-1.41) 0.05 1.23(1.1-1.38) 0.0003 ht4 0.060 0.035 1.26(1.08-1.46) 0.003 ht5 0.000 0.001 . . ht6 0.002 0.000 . . Supplemental table 5. Associations of TAPBP haplotypes with decline of FEV1 on aspirin provocation Global pHaplotype MAF Mean±SD value ht1 0.669 7.27±10.61 ht2 0.199 8.03±11.94 ht3 0.089 9.04±12.94 ht4 0.042 9.03±11.98 ht5 0.0004 3.00 ht6 0.0004 27.00 0.003 Supplemental Table 6. Regression analysis of TAPBP polymorphisms with the decline of FEV1 normalized with total dosages of aspirin Loci C/C C/R R/R Pa Pb Pc rs3106189 601(1.81±8.50) 516(1.65±6.29) 87(1.97±6.56) 0.91 0.78 0.78 rs2239841 766(1.75±7.77) 405(1.32±4.93) 37(2.18±7.61) 0.51 0.38 0.70 rs3106190 607(1.79±8.46) 519(1.65±6.28) 86(1.99±6.59) 0.95 0.80 0.75 rs2071888 541(1.74±8.71) 547(1.77±6.45) 121(1.70±5.63) 0.97 0.94 0.96 rs1059288 543(1.74±8.69) 547(1.77±6.45) 120(1.71±5.66) 0.98 0.96 0.97 1008(1.74±7.74) 189(1.71±5.84) 11(3.47±7.99) 0.71 0.80 0.53 ht3 % fall of FEV1 normalized with total dosages of aspirin used in challenge test was calculated as % fall of FEV1 divided by total dose of aspirin x 100.