userfiles/39/CPE Syllabus 2012
advertisement

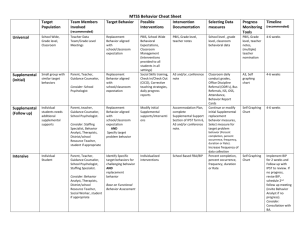
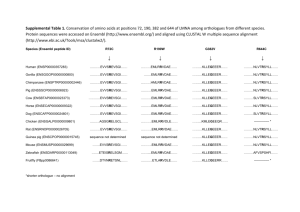
C. P. E Science Course Syllabus Streetsboro High School Streetsboro, Ohio 2012-2013 Instructor: Ms. Amanda C. Hudnall ahudnall@rockets.sparcc.org 330-626-4906 ext 6216 Course Description: C. P. E. Science is a freshman level course that focuses on the major concepts relevant to a comprehensive introduction to the fields of Chemistry, Physics and Earth Science. All major topics covered will be enhanced with laboratory exercises and practical applications. Science skills and the Scientific Method will also be a focus throughout the entire course. The inquiry approach to science will be stressed with emphasis placed on those performance objectives addressed in the Ohio Science Curriculum Standards. Completion of all homework assignments, class work and laboratory reports is expected of all students. Tentative schedule of topics: Week 1 Text Correlation, Ohio Science Standards Ch I (p 6 – 29) Topic(s) Introduction to Laboratory Practices, Scientific Methods. The Nature of Science Scientific Inquiry (A1-6) 2 3 Scientific Ways of Knowing (A1-3) Ch III ( p 70-89) , Standards of Measurement, Graphing Skills Motion: Description of Motion, Acceleration, Velocity, Motion in terms of Force, Friction Physical Science (D 21 – 25) 4 Ch IV ( p 98 – 119) Newton’s Laws ( I, II, II) 5 Physical Science (D 21 – 25) Practical applications Gravity Ch 7 (p 184 – 209) The Earth in space Time and the Seasons The Moon and it’s cycles Supplemental Material 6 Ch 8 (p 216 – 243) Supplemental Material 7 8 9 10 Ch V (p 128-145) Ch IX (p 252-279) Physical Science (E1213) (F3) , (F11) (F15) (F17) Ch IX (p 252-279) Physical Science (E1213) (F3) , (F11) (F15) (F17) Ch V Section II ( p135145) Ch XXV (p784 – 810) Ch XXV (p784 – 810) Planetary Motion The Inner Planets and their orbits The outer planets What is Energy? , Thermal Energy in terms of Molecular Motion, Energy Transformation within a system, Thermal Energy Transfer, Kinetic Energy, Potential Energy, Gravity (GPE), Conservation of Energy Theorem, Radioactivity, Radiometric Dating Fission vs. fusion reactions, Endothermic vs. Exothermic chemical reactions Physical Science (F3) , (F14), (F16) 11 Ch XV (p454 – 478) 12 Physical Science (G 18 – 19) Ch XI (p 318 – 346) Transfer of Energy – Waves (properties and behavior) Sound, seismic, water and light waves Electromagnetic Spectrum Transfer of Energy – Waves (properties and behavior) Sound, seismic, water and light waves Electromagnetic Spectrum 13 Ch 6 (p 152 – 177) Physical Science (G 18 – 19) Work Simple vs. complex machines 14 Ch XVIII (p550 – 570) Matter – Properties and measurement 15 16 17 Physical Science (G 18 – 19) Properties of Atoms Ch ( 11 (p 318- 345) Sound and Light Supplemental Material The Electromagnetic Spectrum Physical Science (G 18 – 19) Ch ( 11 (p 318- 345) Applicable uses of various types of EM Radiation Supplemental Material Career exploration related to radiation Physical Science (G 18 – 19) Ch ( 11 (p 318- 345) Mirrors, lenses and the eye Supplemental Material Integration of Anatomy with Physical Science Physical Science (G 18 – 19) 18 Review for Midterm Exam Mid Term 19 Ch 18 (p550-570) 20 Physical Science (C9) Ch 18 (p550-570) 21 Physical Science (C9) Supplemental Material 22 Ch 16 (p 486 – 508) Ch 19 (p576 – 600) 23 Physical Science (A1 – 4) Ch 19 (p576 – 600) Properties of Pure Substances Mixtures Colloids Electrical Conduction of Electrons Energy sources and Production Alternative Energy Sources Atoms/Molecules/Elements/ Isotopes General Intro to Chemical Science The Periodic Table Supplemental Material 24 Physical Science (A1 – 4) Ch 22 ( p686 – 712) Ions, Ionic charges, Electrical force between atoms, chemical formulas, chemical equations Physical Science (A5) , (B6), (B7) 25 Ch 23 (p718 – 744) 26 Physical Science (B7) Ch 23 (p718 – 744) 27 Physical Science (B7) Ch 24 (p750 – 778) 28 Physical Science (B8) Physical Science (B8) Ch 24 (p750 – 778) 29 Physical Science (B8) Ch 24 (p750 – 778) 30 Ch 25 ( 784 – 809) Supplemental Material 31 Ch 26 (p816-842) 32 33 Earth and Space Science (A1,2) (B4) Ch 26 (p816 – 841) Supplemental Material Earth and Space Science (A1,2) (B4) Supplemental Material Earth and Space Science (B4) (E5)(E6)(E7) 34 Supplemental Material 35 Earth and Space Science (F8) Supplemental Material 36 Covering ALL Physical Balancing Chemical Equations Atomic Reaction Part I Chemical Changes Classification of Reactions Atomic Reactions Part II pH Scale Solution Formation Acids Bases and Salts Solubility and Concentration Strength of acids and bases Determining solubility Nuclear change; Radioactivity Nuclear reactions Nuclear Reactions in stars as a source of energy Stellar Processes leading to formation of elements beyond H and He Current evidence for the Big Bang Stars and Galaxies Constellations Processes within Earth’s systems and surface Plate Tectonics Sea-floor spreading Continental Drift Historical Perspectives and Scientific Revolutions (Heliocentrism, Plate Tectonics, Nuclear Energy) Exploring Careers related to Chemistry, Physics and Geology Review for Final Exam Science Standards for 9th Final grade students Class Policies: Your regular attendance and participation in classroom activities and discussions is expected. Your success in this course is heavily reliant upon your active engagement in daily classroom activities. Tardiness to class will not be permitted. Please refer to student handbook for tardy policy. Assignments and homework are due at the BEGINNING of the class period for which they are due. Assignments turned in after the start of class will be counted one day late according to the departmental late-work policy (below). Any homework found to be completed during class time will NOT receive credit. Late work policy for Streetsboro High School Science Department 2012-2013: 9th grade students: Homework or work to be collected is due when the teacher announces that it is being collected or at the beginning of the period of a previously announced due date. Collection announcements will be made verbally or through prior posting. Work will be deducted 1/3 credit for each day late. Ex: Original assignment is worth 15 points; 1 day late the maximum points possible would be 10 2 days late the maximum points possible would be 5 3 days late the maximum points possible would be 0 In accordance with the student handbook plagiarism will not be tolerated. Test, quiz, homework, labs, etc. will all be handled the same. The student will receive a zero for the assignment and be issued one afterhours restriction.