Rough Draft Senior Project
advertisement

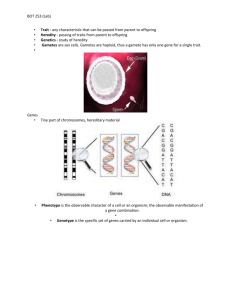
Senior Project Eboni D. Chambers Student Demographic- Eboni D. Chambers I hail from the city of brotherly love and was raised with a strong sense of community, accountability, and emphasis on education. Growing up in Philadelphia, I developed an early interest in the sciences, and often enjoyed visiting the Franklin Institute of Philadelphia as a child and throughout high school. Although I developed an early interest in the sciences, my exposure to successful minority scientists was limited. In the junior year of my high school career, I participated in the Fattah Conference of Higher Education. It was this gathering of successful and influential minority scientists that exposed me to the vast opportunities and exciting careers in the sciences. The conference also allowed me to witness the disproportionate number of minorities in the sciences which further inspired me to pursue a career in biomedical research. I was encouraged to major in biology for my undergraduate studies, a goal which I achieved when I received admission to North Carolina Agricultural and Technical State University (NCATSU). While a student at NCATSU, I have worked diligently to seek opportunities to further increase my exposure to the biomedical sciences and to participate in undergraduate research. To date I have participated in four biomedical research internships. These internships have helped me gain a realistic understanding about the research process and have allowed me to master many laboratory techniques that will be of value throughout my graduate education and scientific career. Furthermore, I have had the opportunity to work in different research environments, thus boosting my confidence and desire to become an independent researcher. In addition to pursuing my goal to become a competent researcher, I have also been a very active student at NCATSU and strived to be a well rounded scholar. I am currently a member of the Student Government Association in which I serve as the senior class secretary. Moreover, I serve as the Community Director of Ignite Greensboro, a student-run student organization that serves as a medium for college students to be effective catalysts of change and competent representatives of progress in our society. I have also been a volunteer with the Girl Scouts of America since 2010. I have been an academic success coach/tutor for the honors program at NCATSU and I have also been on the Deans’ list for multiple semesters. Although I have had exposure to various areas of research, my specific research interests include studying environmental and demographic attributes that contribute to hormone dependent cancers and infectious diseases. Many environmental factors have attributed to minorities being disproportionally predisposed to aggressive cancers and specific gene variants may play a role. I plan on matriculating to Brown University in the fall semester to study Pathobiology. I believe Brown University will facilitate my goals by preparing superb graduate education and thus fulfilling obligations to myself and ultimately society. Table of Contents Abstract- pg 5 Introduction- pg 6 References-pg 8 Abstract Drosophila melanogaster is a powerful genetic model for studying of cancer cell pathways and tumor suppressor genes. The six hallmarks of cancer are self-sufficiency in growth signals, insensitivity to growth-inhibitory signals, evasion of apoptosis, limitless replicative potential, sustained angiogenesis, and tissue invasion and metastasis. Genetic manipulation in D. melanogaster allows for the rapid identification and characterization of genes involved in these six key processes. Our group recently identified L.4.1 as a novel homozygous recessive point mutation that produced overgrowth phenotypes by blocking apoptosis in the adult mosaic eye of D. melanogaster. The studies utilize a conditional growth suppressor screen that used a block in cell death. Combined use of D. melanogaster and inactivation of the apoptosis pathway through mutations in the dark82 gene makes it possible to observe phenotypes from novel homozygous recessive point mutations in flies that would otherwise induce apoptosis. The objective for the present study was to characterize the role of L.4.1 in cell growth regulation, mitosis, and differentiation pathways through genetic mosaics. Specific protein markers were used to analyze differences in the described pathways between mutant and wild-type L.4.1 eye discs. Confocal microscopic analysis was used for illumination of specific protein levels between mutant and GFP marked homozygous wild type tissue within the mosaic eye. Compared to wild-type, L.4.1 mutants had a significant decrease in DIAP staining, suggesting that mutant L.4.1 overgrowth is conditional upon a block in apoptosis. Clones of dark82, L.4.1, and eye discs entirely mutant for dark82, L.4.1 demonstrated a significant delay in differentiation. This was confirmed by a corresponding decrease in the Elav protein; a marker for cell differentiation. This data suggests that the delay in differentiation has a correlation with prolonged cellular proliferation within the cell. The D.melanogaster genome contains many human orthologs which function in similar cellular pathways and processes. Genetic mapping of the L.4.1 gene could thus be a valuable tool in studying genes that play a role in the development of human cancer. Introduction Drosophila melanogaster is a simple yet highly efficient model organism for the study of cancer cell pathways and tumor suppressor genes. Cancer is described by self-sufficiency in growth signals, insensitivity to growth-inhibitory signals, evasion of apoptosis, limitless replicative potential, sustained angiogenesis, and tissue invasion and metastasis (Weinberg 2000). Genetic manipulation in D. melanogaster allow for the rapid identification and characterization of genes involved in these six key processes. Studies correlating tumorigenesis in flies and humans can further the understanding of human cancer development as the D. melanogaster genome contains many human orthologs which function in similar cellular pathways and processes (Potter CJ 2000). Mutations in tumor suppressor genes are associated with many human cancers and disorders, many of which can be expressed in the drosophila model (Huang He 1999). Previous studies have characterized the Drosophila PTEN gene and present evidence that suggests that both inactivation and overexpression of PTEN affect cell size, while overexpression inhibits cell cycle progression at early mitosis and promotes cell death during eye development (Freeman 1996). These specific pathways have led to overgrowth phenotypes within tissues of interest, specifically inside the larval eyes and wings of Drosophila melanogaster4 (Freeman 1996). Studies in the larval eye disc of drosophila melanogaster have contributed to our understanding of differentiation and have promoted our knowledge of differentiation furrows. Ommatidial differentiation begins in the posterior of the eye imaginaldisc, from which point a furrow sweeps across the disc, leaving a trail of ommatidial rows (Freeman 1996). A delay in such differentiation would suggest ommitidial cells to grow exponentially with lack of growth suppression signals; specifically Notch. Notch signaling controls proliferation and differentiation across animal species. Notch signaling is under tight cell regulation and has been found to be reduced in mutant genes (Thomas Vaccari 2008). A previous study in our lab identified genes that restrict cell numbers and tissue growth during development. We conducted a genetic screen to identify recessive mutations that gave homozygous mutant cells even a subtle proliferative advantage over their wild-type neighbors (Tapon 2001). Clones of homozygous mutant tissue were generated in the eyes of heterozygous flies and their size was compared with the wild-type twin spots generated from the same recombination events (Kenneth H. Moberg 2001). We retained flies in which there were more mutant than wild-type tissue through inducing flies with ethylmethylsulfate (EMS); a carcinogen, to produce point mutation in the flies. Of more than 23 loci identified in the screen, we obtained multiple alleles of homologs of several known human tumor-suppressor genes, including TSC1 ,TSC2 and PTEN . A previously unknown locus, represented by a lethal complementation group consisting of three alleles, was named archipelago(ago )(Kenneth H. Moberg 2001). Advancing the research performed previously, our group recently identified L.4.1 as a novel homozygous recessive point mutation that produced overgrowth phenotypes by blocking apoptosis in the adult mosaic eye of D. melanogaster.Our studies utilize a previous conditional growth suppressor screen that used a block in cell death. Combined use of D. melanogaster and inactivation of the apoptosis pathway through mutations in the dark82gene makes it possible to observe phenotypes from novel homozygous recessive point mutations in flies that would otherwise induce apoptosis. The objective for the present study was to characterize the role of L.4.1in cell growth regulation, mitosis, and differentiation pathways through genetic mosaics. References Freeman, M. (1996). "Reiterative Use of the EGF Receptor Triggers Differentiation of All Cell Types in the Drosophila Eye." Cell 87: 651-660. Huang He, P. C. J., Tao Wufan (1999). "PTEN affects cell size, cell proliferation, and apoptosis during Drosophila eye development." Development 126: 5365-5372 Kenneth H. Moberg, D. W. B., Daniel A. Haber, Iswar K. Hariharan, and Doke C. R. Wahrer (2001). "Archipelago regulates Cyclin E levels in Drosophila and is mutated in human cancer cell lines." Nature 413(6853): 311-319. Potter CJ, T. G., Xu T (2000). "Drosophila in cancer research, An expanding role." Trends in Genetics 16(1): 33-39. Tapon, N., Ito, N., Dickson, B. J., Treisman, J. E. &Hariharan, I. K (2001). "The Drosophila tuberous sclerosis complex gene homologs restrict cell growth and cell proliferation." Cell 105: 345-355 Thomas Vaccari, H. L., Rita Kanwar, Mark E. Fortini and David Bilder (2008). "Endosomal Entry Regulates Notch receptor activation in Drosophila melanogaster." Journal of Cell Biology 180(4): 755-762. Weinberg, D. H. a. R. A. (2000). "The Hallmarks of Cancer." Cell 100(1): 57-70.