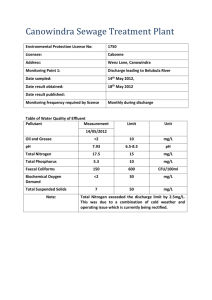
Supplementary Material
advertisement

Supplementary Material for “A Versatile, Pulsed Anion Source Utilizing PlasmaEntrainment: Characterization and Applications” Yu-Ju Lu, Julia H. Lehman, and W. Carl Lineberger JILA and Department of Chemistry and Biochemistry, University of Colorado, Boulder, CO 80309 I. Detailed Schematics of the Anion Source The Supplementary Material provides additional details of the new pulsed ion source, including a photograph of one of the valve setups in this laboratory (Fig. S1). An additional ion source in a separate setup in this laboratory uses an XYZ translation stage to assist in positioning the discharge valve relative to the main expansion (not shown). Given the performance sensitivity to relative positions of the two pulsed valves, as discussed in the main text, this is a highly recommended update. Schematics of the pieces that were machined are shown in Figs. S2-S6, which include dimensions and any cleaning instructions beyond typical maintenance common to pulsed Series 9 General Valves. The machined pieces for this new source include the custom main valve faceplate (Fig. S2), the two electrodes involved in the discharge (Figs. S4 and S6), and the MACOR plate insulators (Figs. S3 and S5). II. Additional Proof-of-Principle Experiments A. H2-tagging: CH3O–(H2)n Beyond solvation of atomic anions (not discussed) and diatomics, this new source successfully attaches one Ar atom and up to three H2 molecules on CH3O–, yielding CH3O–(Ar) and CH3O–(H2)1-3 clusters. This source sufficiently cools the many internal degrees of freedom afforded by this polyatomic molecule to allow for weakly bound clusters to be formed. While not useful for the photoelectron spectroscopy performed in this laboratory, H2 tagging is 1 advantageous for vibrational predissociation spectroscopy, mentioned in the main text, and is shown here as a proof-of-principle to demonstrate the versatility of this source. Figure S7 shows a comparison between two mass spectra for the production of CH3O– (m/z = 31). The CH3O– is formed in the side discharge source (–800 V, 140 μs) from the expansion of Ar gas (15 psig) bubbled through liquid CH3OH (99.8%, MacronTM Chemicals) kept at room temperature. The plasma is then entrained into a main expansion of either neat Ar (black) or a mixture of 25% H2 in Ar (red) at a backing pressure of 40 psig. In the neat Ar expansion, CH3O–(Ar) is formed (not shown) and its identity confirmed by photoelectron spectroscopy. When H2 is added to the main expansion, the intensities at m/z = 33, 35, and 37 increase, denoting the formation of CH3O–(H2)1-3 clusters. When optimizing for the cluster intensities, the intensity of m/z = 31 is about 20 times stronger than the m/z = 33 intensity. B. Tagging Solvated Species: O3–(H2O)nArm The ease with which this source produces anions complexed with weakly bound solvation species, namely Ar, implies that anions with more strongly bound solvents should be readily produced. As described in Ref. 1, this is certainly the case with production of O3–(H2O)n. A 60% O2/Ar gas mixture is flowed over 30% H2O2 and then discharged using -500 V, 100 s width, and an aluminum cathode. The plasma produced is then entrained in a neat Ar main expansion and readily generates O3–(H2O)n clusters. Although H2O is introduced through the side expansion and its high vapor pressure likely results in an abundance of H2O, this does not interfere with the cooling and stabilizing abilities of this anion source. Moreover, these clusters are also seen to be Ar-tagged, O3–(H2O)nArm, again demonstrating the versatility of this source to produce sufficiently cold anionic species as to multiply solvate the cluster, with both a more 2 strongly bound (H2O) and weaker bound (Ar) solvent species. This trait of the new ion source is particularly useful for vibrational predissociation spectroscopy to determine the geometry of an ion within a solvent structure. C. Exotic Species: CH3– This new ion source is also capable of producing unique anionic species. Using conventional pulsed anion sources, it is extremely challenging to produce CH3–, particularly from removing a proton from methane. Methane is an extraordinarily weak acid with an extremely high gas phase acidity. As demonstrated in Refs. 2,3, however, this pulsed ion source provides a straightforward way for making cold CH3–. In 1978, Ellison et al. successfully measured the photoelectron spectrum of CH3– (formed in a discharge) and determined the EA of the methyl radical.4 The CH3– spectrum revealed an extensive progression in the out-of-plane bending (umbrella) mode of the methyl radical in its electronic ground state. However, the low signal-tonoise ratio due to small ion intensities, along with lower resolution than is currently possible, made this experiment and the vibrational assignments quite challenging at the time. In the current experiment, a pulsed electrical discharge (-900 V, 140 μs) of neat CH4 gas takes place in the side pulsed valve; the main expansion is neat Ar (40 psig). Interestingly, CH3– anions were not observed using a dilute gas mixture of 3% CH4 in Ar in the discharge; see Fig. S8. Instead, discharging neat CH4 was necessary for CH3– production, demonstrating the importance of the choice of gas mixtures used in these experiments. The photoelectron spectra of CH3– and further discussion of this system can be found in Refs. 2,3. D. Additional Rational Anion Synthesis: CH3OO– 3 The CH3OO– anion has been previously investigated using a proton abstraction reaction where CH3OOH was added downstream of OH– in a flowing afterglow ion source.5 In the new pulsed anion source, CH3OO– is generated from an association reaction between O2 and CH3–. The CH3– reactant, produced in the side discharge pulse (-900 V) from 20 psig of neat CH4, is entrained into the main expansion of 1% O2 seeded in Ar at a total backing pressure of 40 psig. The CH3OO– adduct is formed and efficiently cooled by Ar in the main supersonic jet. When optimizing conditions for the CH3OO– anion signal, the intensity ratio of CH3– to CH3OO– is 3:1. A photoelectron spectrum at 532 nm (2.330 eV, not shown) confirms the identity of m/z = 47 following a comparison with that previously obtained from the flowing afterglow photoelectron spectrometer using 363.8-nm radiation.5 Again, the generation of CH3OO– demonstrates the diverse capabilities of this novel pulsed anion source. The reaction of CH3– with O2 to generate CH3OO– is exothermic by 2.37 eV.5 Despite the substantial exothermicity, this reaction still occurs and the hot anion is sufficiently cooled by the Ar carrier gas in the main expansion to result in a stable, isolated anion with no vibrational hot bands in the photoelectron spectrum. This cooling capability is particularly interesting in this proof-of-principle experiment since the side expansion is a neat polyatomic gas (CH4). One might think that this should inhibit any cooling taking place, but because the hot neutral molecules are not significantly affecting the main expansion, this is not the case. Hence, this pulsed anion source performs largely exothermic rational ion synthesis to make transient species that are internally cold. It is worth noting that additional pulsed valves placed further downstream of the main expansion could be used for the sequential addition of neutral reactants to cold ions, further expanding the flexibility of this new pulsed ion source. 4 References 1 J. H. Lehman and W. C. Lineberger, J. Chem. Phys. 141, 154312 (2014). 2 Y.-J. Lu, Ph.D. thesis, University of Colorado, 2014. 3 Y. J. Lu, A. Maple de Oliveira, and W. C. Lineberger, Manuscript in preparation (2014). 4 G. B. Ellison, P. C. Engelking, and W. C. Lineberger, J. Am. Chem. Soc. 100, 2556 (1978). 5 S. J. Blanksby, T. M. Ramond, G. E. Davico, M. R. Nimlos, S. Kato, V. M. Bierbaum, W. C. Lineberger, G. B. Ellison, et al., J. Am. Chem. Soc. 123, 9585 (2001). 6 K. Luria, W. Christen, and U. Even, J. Phys. Chem. A 115, 7362 (2011). 5 Figure S1. Picture of the dual valve setup showing how both pulsed valves are mounted within the source chamber. This picture also displays how the stack of insulators and electrodes are held together. All components of the discharge device have four bolt holes and are fastened onto the faceplate of the side solenoid valve using nylon screws. 6 Figure S2. Dimensions of the main expansion modified faceplate. As discussed in the main text, in order to increase the forward intensity of the main expansion, a 40° conical nozzle with a 0.5-mm orifice (DO) and an aspect ratio (L/DO) of 7 was machined.6 The nozzle seals in the same fashion as a commercial General Valve faceplate, with matching internal threads and o-ring groove. 7 Figure S3. Dimensions of the final MACOR plate. This MACOR plate separates the pulsed negative voltage electrode (Figure S4) from the body of the main pulsed valve (Figure S2). 8 Figure S4. Dimensions of the electrode (S.S. or aluminum) pulsed to negative high voltage. A 40° conical nozzle with a 1-mm orifice is used to collimate generated plasma. In order to have stable discharge, the edge of the orifice is required to be clean and smooth. The surface is cleaned using #400 sandpaper. The edges are smoothed using electropolishing. 9 Figure S5. Dimensions of the thin MACOR insulator between the two electrodes. The central hole of this MACOR insulator is quick to get dirty since the discharge occurs at this region. We use sand “wires” or a small drill to get rid of thin layers from the surface of the middle hole. 10 Figure S6. Dimensions of the thin electrode (S.S.) held at earth ground. This plate is used to avoid heating up or discharging to the faceplate of the pulsed general valve. This plate is also quick to get dirty and damaged, so the surface of the electrode needs to be clean and smooth. As with the other electrode, the surface is cleaned using #400 sandpaper and electropolished to smooth the surface. 11 Figure S7. Comparison of the CH3O–(H2)n=0-3 TOF mass spectra from experiments using neat Ar (black) and 25% H2/Ar (red) for the main expansion. The CH3O– is produced in the side discharge source. 12 Figure S8. Time-of-flight mass spectrum from experiments using (a) 3% CH4/Ar and (b) neat CH4 in the side discharge source. Neat Ar gas is used as the main expansion for both experiments. The chemical identity of the mass-selected anions is confirmed by comparing their photoelectron spectra with other known anion photoelectron spectra. The appearance of F– comes from chemical contamination in sample line. 13