Period: ___ Liver Enzyme Lab Observing the Effect of Temperature
advertisement

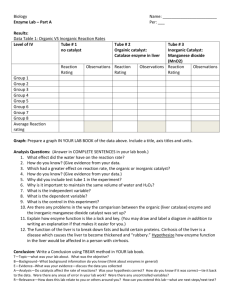
Name: __________________________ Date:___________ Period: ___ Liver Enzyme Lab Observing the Effect of Temperature on Enzyme Activity Outline of Graded Work: To prepare for this lab you will need to read through the lab and complete the following on your own paper. See last page for lab report guidelines. Pre-Lab and Questions: You are a research assistant at a major university. Recently, you witnessed a demonstration that sparked your interest in controlling the rate of reactions. During the demo, a professor used manganese dioxide (MnO 2) to break down hydrogen peroxide (H2O2) into water (H2O) and oxygen (O2). MnO2 + 2 H2O2 MnO2 + 2 H2O + O2 Manganese dioxide is an inorganic catalyst. It has the ability to speed up a reaction. H 2O2 will break down naturally in the presence of light. That is why it is stored in a dark brown bottle. However, when MnO 2 is added to H2O2, the rate of the reaction dramatically increases. Pre Lab Questions: Please thoughtfully answer the questions below. Copy & answer in your lab report. 1. In the equation above, label the catalyst, the substrate, and the products. 2. MnO2 is a catalyst. How is that different from an enzyme? 3. How would you know that a reaction had occurred? Background: Organic catalysts are called enzymes. Enzymes are proteins that speed up the rate of chemical reactions that would otherwise happen more slowly. Enzymes will speed up the rate of a reaction by decreasing the energy required to activate a chemical reaction. The enzyme is not changed or consumed during the reaction. There are hundreds of different enzymes in each cell. Each of these enzymes is responsible for one chemical reaction that occurs in the cell. Hydrogen peroxide is a chemical used to treat wounds. It is an effective antiseptic because it is deadly to cells. Of course, the cells that need to be destroyed are bacterial cells that may enter a wound. Note that hydrogen peroxide is produced as a waste product in every cell of the body. It is toxic to cells. The enzyme catalase (which is found in the liver) speeds up the reaction that breaks hydrogen peroxide into two substances—water and oxygen. Water and oxygen are not toxic to the cell. The reaction is as follows: 2 H2O2 + catalase 2 H2O + O2 + catalase Purpose: The purpose of this lab is to: 1. Determine which substance (H2O2 or catalase) is the substrate and which is the enzyme. 2. Determine how temperature affects the rate of reaction. Procedures: Procedure # 1: Setting up the control groups. 1. 2. 3. 4. Place 1 piece of liver in a test tube. Add 2 ml of H2O2 to the test tube and watch for the reaction. Record the rate of the reaction in data table #1. Save the liver and the liquid in this test tube for another experiment. Thinking questions. 1. How did you know that a reaction was occurring? 2. Explain why liver cells have catalase. Procedure #2. Determine which substance (H 2O2 or catalase) is the substrate and which is the enzyme. 1. 2. 3. 4. 5. 6. Pour the liquid from the reaction in procedure 1 above into a different test tube (leave the used liver in the original test tube). Place one piece of new liver in the test tube with the liquid and watch for the reaction. Record the rate of the reaction in data table #2. Add 2 ml of H2O2 to the original test tube with the used liver and watch the rate of the reaction. Record the rate of the reaction in data table #2. Clean out both test tubes in the sink making sure that the liver gets in to the strainer, not into the sink. Wash the test tubes. Dispose of liver into the trashcan. Thinking questions. 1. Where is the catalase located? 2. What is the enzyme in this experiment? 3. What is the substrate in this experiment? 4. What are the products of the reaction? 5. Explain how these procedures lead you to discover which substance was the enzyme and which was the substrate. Procedure #3. Determine how the temperature affects the rate of the reaction. 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. Put a piece of liver into a test tube. Put the test tube into a boiling water bath for 2 minutes and then remove. Add 2 mil of H2O2 to the test tube and watch the reaction. Record the rate of the reaction in data table #3. Clean the test tube as discussed in the last procedure. Add a piece of liver to a test tube. Place the test tube in a cold water bath for 3-5 minutes and then remove. Add 2 ml of H2O2 to the test tube and watch for the reaction. Record the rate of the reaction in data table #3. Clean the test tube. Thinking questions 1. Compare the rate of the reaction in this experiment to the control. At which temperature did catalase work best? Hot, cold, or room temperature? 2. Knowing this enzyme is found in cells in the body, how does this experiment relate to the danger of having an extremely high temperature? DATA: Put an X in the box for each scenario that best describes the reaction rate observed and record the time. Controls Liver & H2O2 Comparison of Rates of Reaction for Catalase in Different Environments Data Table #1 None Slow Moderate Fast Substrate or Enzyme Used liver & new H2O2 Used H2O2 & new liver None Data Table #2 Slow Moderate Fast Temperature Hot Liver Cold Liver None Data Table #3 Slow Moderate Fast Post-Lab Questions 1. What is an enzyme? 2. What is catalase? 3. What is hydrogen peroxide? 4. What substances are produced after the reaction? Why is this break down necessary? 5. Summarize the environment that the enzyme catalase will work best in. 6. Summarize the environment that the enzyme catalase will not work well in. Conclusion: Form a conclusion using the data. Your conclusion is a formal report so it needs to be written in the pass tense and third person. Include the following things in the conclusion: Restate the purpose in your own words Summarize the procedures used in the lab Explain the data and observations Make a conclusion from your data/observations. Was your hypothesis correct or proven wrong? Error Source: In what ways could the data/observations be incorrect? LAB REPORT GRADING FORM Title II. Hypothesis - Written in if – then format (must be testable) - Experimental Design: Identify independent and dependent variables. III. Materials - Listed IV. Procedures - Concise description; can be a numbered list. V. Pre-Lab Questions - Copy the pre-lab questions and answer them completely. Pre-Lab: 25 Points I. Introduction - Background information – explain what enzymes are & their function - Purpose of the lab This section is not in your lab report, but it will be part of your grade. Participation (25) - Follow safety rules - All group members actively engaged V. Data/Results & Questions (25) -Written observations - Data Table (design) organized, readable, complete - Questions and correct answers written VI. Conclusion (25) - Written in third person, past tense - Restate the purpose - Summarize procedure – DO NOT LIST – explain what really happened - Relate data/results to the purpose - Conclusion – What can you conclude from the results? Comments: Final Score _____________