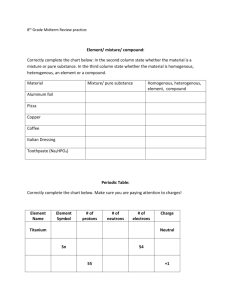
Name: For ionic compounds (bonds between a metal and a
advertisement

Name:____________________________________ For ionic compounds (bonds between a metal and a nonmetal), the metal will have a positive charge equal to its group number for groups 1-3. Transition metals will have their charge listed as a Roman numeral. Nonmetals will have a negative charge (group number minus eight). You must make sure that the charges balance to zero. Example: NaO, sodium has a charge of +1 because it is in group 1, oxygen has a charge of -2 because it is in group 6 and 6-8=0, you need 2 atoms of sodium +1+1=+2 to balance the 1 atom of oxygen -2, +2-2=0, so you will write your answer as Na2O. For covalent compounds (bonds between 2 nonmetals), you do not have to balance the formula. Use the prefixes (mono- = 1, di- =2, tri- = 3, tetra- = 4, penta- = 5) to write the formula. Examples: carbon dioxide = CO2 and dinitrogen pentaoxide = N2O5. For compounds containing polyatomic ions, use the list provided to find the formula and charge of the polyatomic ion. The positive and negative charges must balance to zero. Example: Copper (I) carbonate, Cu has a charge of +1, carbonate is (CO3) with a charge of -2, you need to atoms of copper +1+1=+2 to balance the -2 charge on 1 ion of carbonate, +1+1-2=0, so your will write Cu2(CO3). Use page 173 in your book to see the list of polyatomic ions. 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. What do metals do to become stable? In an ionic compound, what 2 types of ions bond together? Which one is the metal ion in an ionic compound? If there are no nonmetals around to bond with the metal cation, what kind of bond is formed? How do you determine the charge on a nonmetal ion? What charge do alkali metal ions have? Describe metallic bonds. What net charge do metallic bonds have? What charge do alkaline earth metals have? How do you know the charge of the transition metals? Why are these different from the other metals? What determines the strength of metallic bonds? Put these metals in order of increasing metallic bond strength. (Fe, Al, K, Ca) What is the correlation between metal strength and boiling point? What must the net charge of an ionic compound be? Why are metals good conductors? List 3 properties of metals. Why are metals malleable and ductile? What is an alloy? Why is steel strong? What type of elements make up a covalent compound? What type of elements make up an ionic compound? How many elements does a binary ionic compound contain? Write the balanced formula for the following compounds. Titanium (II) sulfide Copper (I) oxide Lead (II) oxide Sodium phosphide Lead (IV) nitride Mercury (II) iodide Nitrogen dioxide Sodium nitride Lithium sulfide Diphosphorus tetrafluoride Carbon dioxide Dihydrogen oxide Dinitrogen tetraoxide Describe a polar covalent bond. Name:____________________________________ 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. What is an ion? Anion? Cation? What metal has the highest boiling point? What do we use it for? What is a molecule? In a covalent bond, electrons are ____________________________. In a HCl molecule, the shared electrons spend more time near the ______ atom than near the _____ atom. Prefixes are used to name which type of compound? When atoms form a polar covalent bond, the atom with the greater attraction for electrons has a partial ___ charge. Which of the following is a polar covalent molecule? Carbon dioxide or water Which has a higher boiling point, polar or nonpolar covalent molecules? Make electron dot diagrams for the following atoms. (C, O, Cl, Mg, K, Xe, I, Ga, Li, H) Identify the following compounds as ionic, covalent, or both. Lead (IV) nitride Mercury (II) iodide Nitrogen dioxide Diphosphorus tetrafluoride Carbon dioxide Ammonium fluoride Zinc (II) dichromate Hg(NO3)2 Name the following compounds. NO2 P2F4 P2O5 CO N2S5 HgO Hg2O Na2O Na(OH) N2O5 H20 Ca(SO4) FeN FeO K2S Al2(SO4)3 Cu2(CO3) N2O4 Ag(NO3) Ca(NO3)2 Al2(CO3)3