Vaishali Patel Lab Report - tran-snc2dd
advertisement

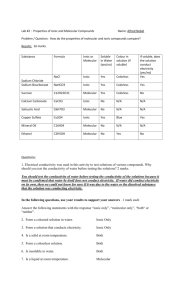
Comparing Ionic and Molecular Substances Lab Report by Vaishali Patel Purpose- comparing the solubility in water and ionic and molecular substances. Hypothesis- If all of the powders have mixed, then it have a reaction. Material- stir rod, watch glass, sample beaker ,distilled water stir rod, conductivity tester, waste beaker, and paper. Watch Glass, stir rod, conductivity tester, sample beaker ,wooden splint, distilled water bottle, waste beaker and paper. ProcedurePut a little bit of distilled water into the watch glass. Take a small amount of the sample and place it on the wooden splint and add water to it. Use the stirring rod to the mix the sample. Pick up the conductivity tester, place it into the watch glass to find if the water and sample mixtures conducts electricity write your observation down into the sheet . Put the mixture into waste beaker. Now wash the watch glass and dry it. Observations Substance Name Type of substance (Ionic & Molecular) Solubility in water Observation Conductivity when testing for conductivity Salt (NaCl) Ionic Partially Dissolved Light on Conduct very well Potassium Chloride (KCl) Ionic Partially Dissolved Light on Conduct very well Copper(II) Sulfate Ionic Dissolved well Light on Conduct very well (CuSO4) Starch Molecular Partially Dissolved Light on Conduct very well Simple Sugar (Glucose) Molecular Partially Dissolved Light on Conduct very well Oil Molecular Partially Dissolved Light off Did not conduct Unknown A Table Sugar Molecular Dissolved well Light off Did not conduct Unknown B (Potassium Bromine KBr) Ionic Partially Dissolved Light on Conduct very well Discussion When Ionic substances are mixed together it partially dissolves but not every substance for example salt and KCl are Ionic substances when they are mixed together it doesn’t dissolved well abut for copper sulfate it dissolved very well maybe it is because of the material is in it. A conductivity substances, when it is dissolved with a sample the light will not might turn on or off it depends. The solubility in water of an molecular substance that can be partially or well dissolved because when it was mixed it wasn’t dissolved right may be because when the powder was mixed with water the material in the powder wasn’t mixed right. The conductively of molecular substance is when the person is puts the conductively tester in the sample and the sample is molecular the light will be off, but if its ionic it will turn on. For unknown A or B if the light turns on it is ionic and if it is not then its molecular, so it dissolved well it is molecular if it didn’t it is ionic. Unknown is A is table sugar because table sugar is molecular when it was tested the light was on ,it dissolved well and it did not conduct. Unknown B is Potassium Bromide because Potassium Bromide is ionic so when a compound is ionic it is partially dissolved and conducted very well. Conclusion- the powder sample was mixed with clean water, whether it was dissolved right, and then record your observation. My hypothesis was wrong because when the experiments were being done there were no reaction nothing was formed. For example artificial snow when you add water the snow gets bigger and bigger.