Kevin Osei`s Ionic and Molecular Substances Lab
advertisement

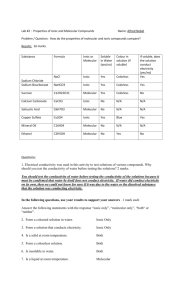
Ionic and Molecular Substances Lab Purpose: The purpose of this lab is to compare the solubility in water, and the conductivity of substances, both ionic and molecular. Hypothesis: In my educated opinion, I would have to assume that the ionic substances will conduct the electricity much better. The reason I say this is due to the fact that ionic substances consist of a metal, and a non metal element. And as you may know, metal is a very strong conductor of electricity. If my hypothesis is correct, this should make the substance very conductible. This would mean that the molecular substances would not have as much as a success rate at conducting the electricity, because it is lacking the metal particles elements. Again, this is just a hypothesis. Dependent/ Independent Variable: In this experiment, the dependent variable would be the powder, because it depends on the water to change it. Therefore, the water is the independent variable. Apparatus: Ionic substances: Salt (NaCl) Potassium chloride (KCl) Copper (II) sulphate (CuSO4) Molecular substances: Glucose (Simple sugar) Starch Oil Watch Glass Stir rod Conductivity Tester Sample Beaker Wooden splint Distilled water Bottle Waste beaker Paper Procedure: 1. 2. 3. 4. Create a toonie sized puddle of distilled water on the watch glass. Place a small amount of the sample substance on the wooden splint, and add to water on glass. Use glass stirring rod to mix the water and the sample. Mix until it is as dissolved as possible. Use the conductivity tester to find out if water and sample mixture conducts electricity. Record observations. 5. Empty mixture into waste beaker, rinse and dry the conductivity tester, watch glass, and stir rod. Observations: Substance Name Salt (NaCl) Potassium Chloride (KCl) Copper (II) Sulfate (CuSO4) Starch Glucose (Simple Sugar) Oil Unknown Substance A Unknown Substance B Type of Substance (Ionic/Molecular) Ionic Ionic Solubility in Water Conductivity Test Observation Conductivity Overall Dissolved well Dissolved well Light on steady Light on steady Very well Very well Ionic Partially dissolved Light on steady Very well Molecular Molecular Partially dissolved Dissolved well Light off Light off Did not conduct Did not conduct Molecular N/A Did not dissolve Dissolved well Light off Light off Did not conduct Did not conduct N/A Dissolved well Light on steady Very well Results: From this experiment, I have received most of the information that was needed. I had taken note that the salt, the potassium chloride, the copper (II) sulfate, and the unknown substance (B) had conducted very well, and all of their lights for the conductivity test were lit steadily. All of those but the copper (II) sulfate had dissolved very well. I had also noticed that the starch, the glucose, the oil, and the unknown substance (A) had not conducted at all, and their light for the conductivity test had not shown whatsoever. It is interesting that the ionic substances had conducted electricity extremely well, and the molecular substances hadn’t, just as I had predicted. Error Analysis: In this experiment, the only thing that could have been a major variable that we could have messed with would be one of two things. Either not cleaning the apparatus well, or not stirring the substances for long enough/ stirring the substances for too long. Discussion: 1. The solubility for the ionic substances was very strong, most of the samples dissolved right into the water. When they are dissolved in the water, from what my observations have shown, the conductivity is extremely strong. 2. For most of the molecular substances, unlike the ionic, most of the substances did not dissolve into the water. Although, when they were in the water, they did not conduct any electricity. 3. Based on my answers to question one and two, I assume that I can use the conductivity properties to determine if the unknown compounds are ionic or molecular by noticing if they conduct electricity at all. If it is an ionic compound, it will conduct, but if it is a molecular compound, it won’t. 4. By my observations, and my knowledge that I have obtained during this lab, I can solve that substance B is ionic, and is the potassium bromide, and substance A is molecular, and also sucrose. Conclusion: From this lab, I have learned that Ionic compounds are very conductible, and usually very soluble, but on the other hand, I have learned that molecular compounds are not conductible, and usually don’t dissolve into water easily. My hypothesis had turned out to be correct.