bananas. Flame tests
advertisement

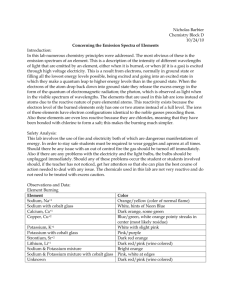
Name:_______________________Hour:______ Coloring the Elements 17 points Part I: Flame Tests Introduction: According to the Bohr atomic model, electrons orbit the nucleus in fixed paths with specific energies. Each path is therefore often referred to as an “energy level”. Electrons possessing the lowest energy are found in the levels closest to the nucleus. Electrons of higher energy are in more distant energy levels. If an electron absorbs sufficient energy to jump between energy levels, the electron may jump to a higher level and become “excited”. Since this results in a vacant lower orbital, the atom is unstable. The excited electron releases its newly acquired energy and falls back to its initial or “ground state” to stabilize Sometimes the excited electrons acquire sufficient energy to jump several levels. When these electrons return to their ground state, several distinct energy emissions occur. Electrons may become excited when a sample of matter is heated or subjected to an electrical current. The energy that electrons emit when returning to the ground state is electromagnetic radiation, or EMR. Sometimes the EMR can be seen as visible light. In 1900, Max Planck studied visible emissions from hot glowing solids. He proposed that light was emitted in packets of energy called quanta and that the energy of each packet was proportional to the frequency of the light wave. According to Einstein and Planck, the energy of the packet could be expressed using frequency () of emitted light and Planck’s constant (h). E=h• As white light passes through a prism its wavelengths are bent at different angles. This makes a rainbow of colors known as a spectrum. If, however, the light emitted from hot gases or energized ions is viewed in a similar manner, isolated bands of color are observed. Each band represents a specific energy level change of electrons in the atoms. Since the atoms of each element contain unique arrangements of electrons with unique energy levels, these bands are unique to each element. They are known as bright line or emission spectra. Flame tests are a common method of determining unknown substances. Recently a skeptic food consumer (we’ll call him Tom) claimed that bananas do not contain significant amounts of Potassium (K). A renowned anonymous scientist refuted Tom’s claim and further claimed that bananas carry not only potassium, but other vital nutrients as well, including sodium (Na). Purpose: Your goal is to try and provide some proof of either claim. In class we discussed how when we burn an element and their electrons absorb energy they will move up to the next energy level further from the nucleus. Eventually, each electron will emit that energy it absorbed and give off a unique light wavelength based on how much energy it absorbed in the first place. Each energy has a different wavelength and is a different color. To accomplish the goal, you will conduct a flame test to try and identify individual substances in a common item: bananas. Flame tests provide qualitative evidence of substances due to distinct flame colors. Question: Are Potassium (K) and Sodium (Na) present in bananas? Hypothesis: Circle one! 1 point If the flame colors of a banana match the K or Na samples, then the banana contains that element. If the flame colors of the K or Na are not observed when a banana burns, then the banana does not contain the element Procedure: follow the safety and lab procedures below. 1. 2. 3. 4. 5. 6. Put on safety goggles and set up Bunsen burner, making sure that all hoses are on tightly USING TONGS, Dip the Q-tip in the rinse water. Dip the Q-tip in the first substance, potassium chloride. Insert the Q-tip into flame but JUST ON THE EDGE. Record the color produced in Data Table 1 Repeat for sodium chloride with a new Q-tip When you have finished obtain a banana chip sample. Perform a flame test and identify the identity of the metals (either potassium, sodium, neither or both) present by the color of the flame. Record your results in the Data 2 and clean up Data Table 1: Record your observations for each substance below 3 points Potassium Chloride Sodium Chloride Banana chip Analysis: Answer each question (using complete sentences) based on your group’s data. 8 points 1. What colors were observed with the banana chip flame test? 2. So what elements can you deduce the banana contains? The electromagnetic spectrum is shown below. Recall that energy is directly proportional to frequency, while frequency and energy are inversely proportional to wavelength. Use this information to answer questions below. 4.0 4.4 4.9 5.5 5.8 6.3 7.0 -5 ( in cm X 10 ) | | | | | | | | violet | blue | green |yellow| orange | red | 3. List the colors observed in this lab from the highest energy to the lowest energy. 4. List the colors observed in this lab from the highest frequency to the lowest frequency. 5. List the colors observed in this lab from the shortest wavelength to the longest wavelength. 6. Was it possible to distinguish potassium from sodium when we burned the banana chip? _____ 7. Why do the chemicals have to be heated in the flame first before the colored light is emitted? 8. Colorful light emissions are applicable to everyday life. Where else have you observed colorful light emissions? Conclusion: Write 5 sentences that address the introduction, answer the question, and support or refute the hypothesis. 5 points Does your data confirm either claim? How do you know? Are potassium and sodium present in bananas? Was your hypothesis supported or rejected?