Study module 5
advertisement

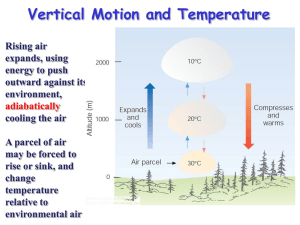
Environmental Monitoring & Technology Series Collect & evaluate meteorological data For Technicians Study module 5 Atmospheric stability cffet.net/env Collect & evaluate meteorological data Study Module 5 Assessment details Purpose This subject covers the ability to site and set up basic ‘ground level’ meteorological equipment and collect and record reliable data. It also includes the ability to assess data quality, interpret significant data features and use the data to ensure the validity of air and noise monitoring measurements. Instructions ◗ Read the theory section to understand the topic. ◗ Complete the Student Declaration below prior to starting. ◗ Attempt to answer the questions and perform any associated tasks. ◗ Email, phone, book appointment or otherwise ask your teacher for help if required. ◗ When completed, submit task by email using rules found on last page. Student declaration I have read, agree to comply with and declare that; ◗ I know how to get assistance from my assessor if needed… ☐ ◗ I have read and understood the SAG for this subject/unit… ☐ ◗ I know the due date for this assessment task… ☐ ◗ I understand how to complete this assessment task… ☐ ◗ I understand how this assessment task is weighted… ☐ ◗ I declare that this work, when submitted, is my own… ☐ Details Student name Type your name here Assessor Marker’s use only Class code CMD Assessment name SM5 Due Date Speak with your assessor Total Marks Available 41 Marks Gained Marker’s use only Final Mark (%) Marker’s use only Marker’s Initials Marker’s use only Date Marked Click here to enter a date. Weighting This is one of seven formative assessments that make up 20% of the overall mark for this unit Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 1 Collect & evaluate meteorological data Study Module 5 Atmospheric stability In Chapter 4, we examined the concept of horizontal and vertical atmospheric motion, and how pressure differences create the winds that form on a variety of different scales. In this chapter, we examine the consequence of the vertical lifting of air; atmospheric stability. As you could predict from the terms, an atmosphere that is stable will usually bring fine weather, and one that is unstable may produce stormy weather. This chapter answers the questions of what constitutes a stable atmosphere and what changes cause a stable system to decompose to an unstable one. Exercise 5.1 Complete the glossary of terms (pg 15) before continuing to help understand this chapter. Setting the scene (the stability toolbox) We now need to apply all of the physical aspects of meteorology that we have learnt from the previous chapters. We will observe how buoyancy (which creates convection) and the horizontal pressure gradient force (hPGF), which creates advection) work together with pressure, temperature and density to create the stable and unstable weather we observe. Equations of state and gas laws To see how all of these come together, we need to explore some of the governing equations, the first of which is called the equation of state. An equation of state Where, at sea level; = atmospheric density in kg/m3 P = atmospheric pressure in kPa R = gas constant T = atmospheric temperature in K Using this equation we can determine the unknown parameter by solving with the three known parameters. In the equation given, we are obviously solving for density, but we can easily rearrange to solve for pressure and temperature. Exercise Solve the above equation for density given a pressure of 99.4 kPa, and a temperature of 306 Kelvin. Rearrange the equation of state above to solve for pressure given a density of 1.18 kg/m3 and temperature of 303 Kelvin. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 2 Collect & evaluate meteorological data Study Module 5 But what does all this mean? The fact that these parameters are related means that if we change one variable, we will change all the others as well, and we need to have a better understanding of what these relationships are. For example, if we change the temperature, does that change the density of the air? The gas laws The gas laws describe the relationships between all of the physical variables that the atmosphere (or gases in general) exhibit. We cannot explore the behaviour of the atmosphere without understanding the basics of how gases work. Some of the relationships have been explored already, but now we need to understand the mechanisms of change by examining some of the calculations. The pressure/height relationship The second significant relationship we need to be reminded of is the pressure/height relationship. The pressure through a vertical slice of the atmosphere is not the same – it follows a curved pattern that decreases with altitude as seen in Figure 5.1 below. Figure 5.1 – Graph showing how atmospheric pressure changes with height. Graph was made in Excel using the Barometric Formula. This is due to the compressibility of air (which creates a pressure gradient), the gravitational force, as well as the pressure gradient force. As you could imagine, physicists have created many formulas for calculating the change in pressure for any given height (see website for spreadsheets). The effect of water You should know by now that water has some very unique properties; a high specific heat capacity, found in all three states of matter, highest density is at 4°C, can easily sublimate under the right conditions, and there are many more. What you may not know is that water plays an enormous role in determining the stability of weather, which implies that Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 3 Collect & evaluate meteorological data Study Module 5 measuring the relative humidity is important for things other than knowing how comfortable (or uncomfortable) the weather will be tomorrow! Let’s begin by understanding how water changes its physical state, i.e. solid, liquid and gas (vapor). The change of state is controlled by temperature, obviously, so solid ice melts to become liquid water at 0°C (the melting point), which boils to become a gas at 100°C (the boiling point). Figure 5.2 below shows the states of matter (assuming water) where the images are depicting the common definitions used when defining the states of matter and how the three states interact with the container that holds them. What you may not know is that water not only changes state from liquid to gas at 100°C but also everywhere in-between, furthermore, solid ice can change into gaseous water vapour without first becoming a liquid! This process is called sublimation (and for our purposes here, we shall assume this is the reason that no atmosphere can be totally dry or free from water vapour). Figure 5.2 – The three states of matter showing how they interact with the container that holds them. Adapted from http://www.grc.nasa.gov But you already knew this, because this non-boiling change of state is called evaporation! This vaporisation of water produces a small partial pressure which is added to all of the other partial pressures exerted by the gases in the atmosphere (all of which are related to the % compositions of each gas). The pressure exerted by vaporised water in the atmosphere is called the vapour pressure of water, and is immensely important parameter in meteorology. Figure 5.3 – Graph showing how water vapour pressure changse with temperature. Note that the vapor pressure of water is equal to that of atmospheric pressure when T = 100°C (i.e.374 K = 760 mm Hg). Graph made in MS Excel using the Clausius-Clapeyron equation. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 4 Collect & evaluate meteorological data Study Module 5 Water evaporates at all temperatures, but obviously more so at higher temperatures (another curved graph!) and again, scientists have formulated many equations to calculate the amount of vapour that comes off water or ice (MetExplore spreadsheet). The vapour pressure of water can have many units, a common one being kPa or some other derivative. One derived unit of measure that you would be very familiar with is called the relative humidity (% RH). The % RH value is a measure of the moisture content as vapour in the air. It can be calculated directly from various equations, but most people are familiar with looking the % RH value up from a humidity table (Appendix A, or MetExplore spreadsheet under the ‘Humidity Table’ tab). Just like vapour pressure, the % RH increases with increasing temperature. So, now that we know that water evaporates with an increase in temperature producing water vapour, and that the most common measure of this water vapour is relative humidity, we can start to look at the reverse situation in which we see water vapour condense out of the air into liquid water. Obviously we need a reduction in temperature for this to occur, and the temperature at which this occurs is called the dewpoint temperature. The dewpoint temperature (Td) The dewpoint temperature effectively asks the question “to what temperature will we need to reduce a parcel of air in order to achieve 100% RH (assuming a given temperature and relative humidity <100 %)”. We do this by calculating the dewpoint temperature. To understand exactly how the air temperature, dewpoint temperature and relative humidity are related, it is best to view a graph of the difference between the two temperatures against the relative humidity (or vice versa). Figure 5.4 – Graph showing the relationship between the dewpoint temperature and the relative humidity. The closer the dewpoint temperature is to the ambient air temperature (i.e. a difference of zero on the x axis in the graph above) the higher the relative humidity. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 5 Collect & evaluate meteorological data Study Module 5 The moisture/density relationship Finally we need to look at how air containing moisture exhibits a change in density, and how this density changes the buoyancy of an air parcel, which will eventually lead to the rising or falling of an air parcel. Figure 5.5 below shows how the density of air decreases as the vapour pressure of water increases, but why is this so! It is due to the fact that the molar mass of air is ~29, but that of water is only 18, so the more water vapor you add, the less dense the air becomes, the more buoyant air becomes. Figure 5.5 – Plot of air density (kg/m3) versus vapour pressure (Pa). An Increase in vapour pressure (or relative humidity) corresponds to a decrease in density as water has a molecular weight of only 18 compared to airs molecular weight of ~29 – a kind of ‘mass dilution’! Lifting Mechanisms in the Atmosphere Applied to the atmosphere, the term ‘stability’ simply means ‘resistant to change’. As mentioned in the introduction, the mention of stability here refers to vertical stability, as any form of sustained vertical motion will generate an unstable atmosphere. This upwards motion causes turbulence in the air, usually rapid turbulence, and combined with the condensation of water due to adiabatic cooling, usually produces some stormy weather, which may or may not produce rain at the surface. To achieve this we need to start ‘lifting’ the air, and this can generally be caused by one (or more) of four mechanisms; orographic lifting, convergence, diabatic heating and frontal systems. Orographic Lifting Orography is a concept in geography (specifically topography) that deals with the height of land. Mountainous terrain (can) provide a natural barrier to horizontal winds, the consequence of which is the vertical motion of air as the wind hits the mountain; it is forced Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 6 Collect & evaluate meteorological data Study Module 5 to go upwards (unless is can go around). The process of a parcel or layer of air rising as a result of the topography is referred to as orographic uplifting. If the parcel or layer of air contains moisture (in the form of vapour – humidity) then as air rise up the side of the mountain, the pressure and temperature decrease adiabatically until it reaches the lifting condensation level (LCL), which is that point in a parcel of air when the pressure and temperature has dropped to the point were relative humidity approximates 100 %, and clouds start to form (which is why the underneath of these types of clouds are generally flat. Figure 5.6 – Orographic lifting. The learning outcome here is two-fold. From a lifting perspective, the lift is created by the topography (the air is forced up by the mountain). The cloud forms due to the resulting instability (vertical movement of air is enhanced – change cannot be resisted!). Image from http://www.waterencyclopedia.com/Ce-Cr/Climate-Moderator-Water-as-a.html Convergence Convergence is another mechanism that can force air near the surface to rise. If winds blowing in different directions meet each other, the different moving air masses become an obstacle to one another. The air converges and has no place to go but upwards. At the surface air flows inward to the center of low pressure where it converges and then rises. Figure 5.7 – Convergence of air parcels resulting in a lifting mechanism of air. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 7 Collect & evaluate meteorological data Study Module 5 Convergence also occurs when air flowing over a smooth surface suddenly hits a rougher surface and slows due to increased friction. The air piles up at the rough surface where the friction is greater, and this causes some of the air to move in a vertical direction. Diabatic Heating The radiation emitted by the earth (which comes from the Sun) heats the air at the surface. Of course both the degree (amount) and rate of heating depends explicitly on the surface that radiates the heat (see albedo). Air that is relatively warm compared to its surrounding rises, and it can (and usually does) cool adiabatically. As a result, the temperature drops in response to the change in pressure as per the laws of adiabatic expansion and compression. Heating that occurs via the sun’s radiation as diabatic heating, which is the opposite of adiabatic. The suns radiation energy is absorbed by matter (air, land, water etc), which results in the convection of warm air. which expands the air, creating a parcel that is of lower density and therefore able to rise vertically through the atmosphere, and is therefore a form of lifting. You can safely assume that this is the most common form of atmospheric lifting. Figure 5.8 – An example of diabatic heating, which effectively results from conduction of radiated energy the sun through matter which results in the convection of heat energy creating buoyant air that rises vertically – causing lift. Image adapted from http://geography.berkeley.edu/programcourses/CoursePagesFA2006/Geog40/L10.pdf Frontal Systems The final lifting mechanism which we will discuss is the overriding of air at frontal boundaries. Moist air, because it is less dense, will override dry air. But how is this different from convergence? In the case of convergence, the lifting results from air molecules pushing one another upward, like pushing two small piles of sand together with your hands, forcing a larger pile to form. When two frontal boundaries meet, the lifting that occurs is due to the relative buoyancy of the two air masses. The more buoyant air mass will override the lesser buoyant air mass. The buoyancy is determined by the characteristics of the air masses (i.e. temperature and moisture content). Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 8 Collect & evaluate meteorological data Study Module 5 Figure 5.9 – Warm and cold fronts. Assume that a cold front ‘cuts’ under warm air, and a warm front ‘rolls’ over cold air. Either way, a parcel of air is lifted. Adapted from www.classzone.com. Adiabatic Processes Think about where we are up to. We now know all of the significant behaviours of the atmosphere; pressure changes, temperature changes, density changes, and the relationships between these three variables; the fact that air is convecting and advecting, as well as the laws of buoyancy and adiabatic expansion and contraction. Of all of these behaviours, the most important or ‘keystone’ variable behaviour is temperature. When air rise and cools (assuming adiabatically), it doesn’t just get suddenly colder, the temperature changes by a certain degree per unit of altitude, which we call a rate. Because we talk about the temperature getting cooler, we refer to the rate of change as the lapse rate, of which there are four key rates to consider; ◗ Dry ◗ Saturated ◗ Environmental, and ◗ Dewpoint The key thing to remember here is that knowing the rate at which rising air cools is vital in determining the stability of the atmosphere. One important distinction should be made at this time; if a lapse rate increases, then the temperature gets colder as an air parcel rises and vice versa. To avoid confusion, we shall suggest in these notes that parcels of air rise and fall, but lapse rate increase or decrease. The Dry Adiabatic lapse rate (DALR) When a parcel of air rises, it expands, and the temperature decreases. Likewise, when air sinks, it compresses, and the temperature increases. When a parcel of air, either dry or containing water vapour (<100%), rises or sinks without the addition or extraction of heat, Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 9 Collect & evaluate meteorological data Study Module 5 that process is said to be a dry adiabatic process. Scientists have determined that this ‘theoretical’ lapse rate is equal to 9.8°C/km (-9.8 if the parcel rises, + 9.8 if it falls). Figure 5.10 – Plot of DALR showing theoretical linear decrease in temperature at a rate of 9.8°C per kilometre. The term ‘lapse’ applies to rising air, ‘gain’ to falling air. Saturated Adiabatic Lapse Rate (SALR) Note that the saturated adiabatic lapse rate (SALR) is also called the moist (MALR), wet (WALR) (sometimes even called pseudo) adiabatic lapse rate. everything that we have learnt so far, it comes as now surprise that colder air contains less water vapour due to the adiabatic and gas laws, so that when a parcel of air reaches its saturation point (dewpoint), condensation begins and eventually clouds will form. Figure 5.11 – Comparison of DALR and SALR. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 10 Collect & evaluate meteorological data Study Module 5 As water vapour condensed to liquid water at the dewpoint, latent heat is given off back to the air parcels environment, thus warming the air. In the reverse process, evaporation, we find that latent heat is taken up from the air parcel, thereby cooling the parcel of air. Since no heat is exchanged between the parcel and the environment, we still refer to this heating and cooling as an adiabatic process. However, during the processes of condensation and evaporation, the cooling and heating of the saturated parcel varies somewhat from the purely dry adiabatic process we discussed above. A rising saturated parcel cools at a slower rate due to the release of latent heat, and a sinking saturated parcel heats more slowly due to the conversion of heat energy during evaporation. This cooling of a rising (or heating of a sinking) saturated parcel is called the saturated adiabatic process which has been calculated to be an average of 6.5OC/km. The figure above shows that the SALR is much less than the DALR. Environmental Lapse Rate (ELR) So, the DALR is a sort of theoretical, calculated ‘moistureless’ lapse rate equalling 9.8OC/km, and the SALR is a warmer lapse rate due to moisture levels approximating 6.5OC/km. So what do we get if we raise a weather balloon in the air and measure the actual temperature of the atmosphere? The easy answer is environmental lapse rate (ELR). The problem is that the atmosphere can really be doing three things when it comes to temperature. The temperature at any point can be in a state of; ◗ Lapse ◗ Isothermal ◗ Inversion Figure 5.12 – The Environmental Lapse Rate (ELR) showing the three possible states of temperature change; lapse, isothermal and inversion. Graph from Excel. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 11 Collect & evaluate meteorological data Study Module 5 We already know that a lapse means a decrease with altitude, so the environmental, or real, atmosphere is getting colder (indicated by a negative slope on a graph). Isothermal means ‘staying the same’, or ‘paused’, and so the temperature remains constant with altitude (indicated by a vertical line on the graph). Yet sometimes we can see an increase in temperature with an increase in altitude, and this is called a temperature inversion. These three states can be seen on the hypothetical plot in Figure 5.12 above. The Dewpoint Adiabatic Lapse Rate (DPLR) Even though a parcel of air may contain moisture, if the parcel is rising, then it cools according to the dry adiabatic lapse rate until it reaches the dew point temperature, Td. We refer to the pressure where the actual temperature equals the dew point temperature as the Lifting Condensation Level (LCL). At the LCL, the cooling process becomes a moist or saturated adiabatic process. Stable, Neutral, and Unstable Atmospheres The stability of the atmosphere is basically determined by comparing the lapse rate of a parcel of air to the lapse rate of the surrounding air, which we also refer to as the environment. If we know the temperature and dew point of the air parcel before it begins to rise, then we can pretty accurately determine the temperature change as it rises. Figure 5.13 – Stable, conditional and unstable atmospheres as shown by comparing three different environmental lapse rates (ELR’s E1,2,3) to both the DALR and the SALR. Measurement data provides us with a profile of the environment with which to compare our rising air. But, keep in mind, our profile is merely a snapshot of the atmosphere. The measurements only give us a view of the atmosphere at a point in time. Motion in the atmosphere makes these comparisons complicated because motion causes changes in the lapse rates we wish to compare. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 12 Collect & evaluate meteorological data Study Module 5 We have found that there are three basic categories in which the atmosphere or a layer in the atmosphere can be classified in terms of stability. These categories, which we discuss next, are stable, unstable and conditional. The Stable Atmosphere (& Neutral) As previously mentioned, a stable atmosphere is strongly resistant to changing its vertical position (regardless of the height of the atmosphere), so if there was some force trying to push the air up or down, in a stable atmosphere this ‘push’ would be resisted, and the air parcel, if pushed, would move back to where it came from. Reference 2 states that; “In a stable atmosphere, if you lift a parcel of air, the temperature of the rising air will decrease fast enough that its temperature will always be colder than the temperature of the environment. Colder air sinks. If the force pushing the air up suddenly disappeared, the parcel would sink back down to its original position where its temperature and pressure would be in equilibrium with the environment.” This is indicated by figure 5.13 above. Absolute stability occurs when ELR is less than SALR (and therefore less than DALR). This means that ELR must be lower than SALR (which varies between 3.9ºC/1km and 7.2ºC/1km) which will never be more than 7.1ºC/1km (if SALR is at its maximum). Neutral stability occurs when ELR and DALR are equal. That is, when ELR is 9.8ºC/1km. It is called 'neutral' because the thermal keeps its initial momentum and does not accelerate or slow down. This is not indicated in figure 5.13. The Unstable Atmosphere An unstable atmosphere is one where a parcel or layer of air is encouraged to rise or fall. In an unstable layer, the lapse rate of a rising parcel is less than the lapse rate of the environment. Because the parcel is warmer than the environment, the parcel has positive buoyancy and continues to rise on its own. Absolute instability occurs when ELR is greater than DALR. We have learned that DALR is 9.8ºC/1km, therefore we can conclude that absolute instability exists when ELR is 9.9ºC/1km or greater. Meteorologists call this a "super-adiabatic lapse rate" since heat loss with elevation is so rapid. Conditional Instability To determine if the atmosphere is conditionally unstable, we compare the lapse rate of a parcel to the environment as it passes through the LCL. The atmosphere is described as conditionally unstable if the environmental lapse rate is less than the dry adiabatic lapse rate beneath the LCL and greater than the saturated adiabatic lapse above the LCL. So, conditional instability can be easily determined by making a comparison of all three lapse rates. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 13 Collect & evaluate meteorological data Study Module 5 Conditional Instability occurs when ELR is less than DALR but more than SALR. In other words, it is when ELR is between SALR (which varies between 3.9ºC/1km to 7.2ºC/1km) and DALR (which is 9.8ºC/1km). The 'condition' for instability is only when the thermal becomes saturated, not before. Conclusion Atmospheric stability is not an easy concept to absorb. Overall, meteorologists use weather balloons to measure the vertical layers of the atmosphere for many parameters including temperature and pressure. From this data they produce one of four common thermodynamic diagrams to examine the atmospheres stability (SkewT-LogP, Tephigrams, Stuve Diagrams and Emagrams). Appendix B is an example of one of these diagrams from the BoM. Good luck with that. What You Need To Be Able To Do ◗ Be able to understand the significance of equations of state ◗ List and describe the relevance of the Gas Laws ◗ Explain the causes of the relationship between pressure and altitude ◗ Explain vapor pressure and humidity ◗ Explain the relationship between humidity and temperature ◗ Define the term Dewpoint Temperature and explain its relevance ◗ Discuss the relationship between humidity and air density ◗ List and describe four major mechanisms which lift air. ◗ List and describe the four major lapse rate ◗ Discuss the three common atmospheric stability scenarios Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 14 Collect & evaluate meteorological data Study Module 5 Assessment Task After reading the theory above, answer the questions below. Note that; Marks are allocated to each question. Keep answers to short paragraphs only, no essays. Make sure you have access to the references (last page) If a question is not referenced, use the supplied notes for answers Complete the following table of important terms. 0 mk Term Definition Atmospheric stability Type your answer here. Equation of state Type your answer here. Evaporation Type your answer here. Partial pressure Type your answer here. Relative humidity Type your answer here. Dewpoint temperature Type your answer here. Density Type your answer here. Orographic lifting Type your answer here. Convergence Type your answer here. Diabatic heating Type your answer here. DALR Type your answer here. SALR Type your answer here. ELR Type your answer here. DPLR Type your answer here. Stable Type your answer here. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 15 Collect & evaluate meteorological data Study Module 5 Unstable Type your answer here. Neutral Type your answer here. Conditional stability Type your answer here. Atmospheric stability Type your answer here. Equation of state Type your answer here. Evaporation Type your answer here. Partial pressure Type your answer here. Answer the following questions a) What information can an equation of state tell us? 4 mk Type your answer here Leave blank for assessor feedback b) What relationship does Boyles Law state? 1 mk. Type your answer here Leave blank for assessor feedback c) What relationship does Charles Law state? 1 mk Type your answer here Leave blank for assessor feedback d) What relationship does the Gay-Lussac Law state? 1 mk Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 16 Collect & evaluate meteorological data Study Module 5 Type your answer here Leave blank for assessor feedback e) What relationship does Avogadro’s Law state? 1 mk Type your answer here Leave blank for assessor feedback f) State how and why pressure changes with altitude? 5 mk Type your answer here Leave blank for assessor feedback g) What is vapor pressure? 2 mk Type your answer here Leave blank for assessor feedback h) What is the relationship between water vapor and humidity (trick question?) 1 mk Type your answer here Leave blank for assessor feedback i) What is partial pressure? 2 mk Type your answer here Leave blank for assessor feedback Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 17 Collect & evaluate meteorological data Study Module 5 j) How does vapor pressure change with temperature? 2 mk Type your answer here Leave blank for assessor feedback k) What is the dewpoint temperature? 2 mk Type your answer here Leave blank for assessor feedback l) What is the relationship between the dewpoint temperature and humidity? 2 mk Type your answer here Leave blank for assessor feedback m) Describe the relationship between humidity and air density. 3 mk Type your answer here Leave blank for assessor feedback n) List and describe the four main lifting mechanisms that can occur in the atmosphere. 8 mk Type your answer here Leave blank for assessor feedback o) List and describe the three common atmospheric stability scenarios. 6 mk Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 18 Collect & evaluate meteorological data Study Module 5 Type your answer here Leave blank for assessor feedback Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 19 Collect & evaluate meteorological data Study Module 5 Assessment & submission rules Answers ◗ Attempt all questions and tasks ◗ Write answers in the text-fields provided Submission ◗ Use the documents ‘Save As…’ function to save the document to your computer using the file name format of; name-classcode-assessmentname Note that class code and assessment code are on Page 1 of this document. ◗ email the document back to your teacher Penalties ◗ If this assessment task is received greater than seven (7) days after the due date (located on the cover page), it may not be considered for marking without justification. Results ◗ Your submitted work will be returned to you within 3 weeks of submission by email fully graded with feedback. ◗ You have the right to appeal your results within 3 weeks of receipt of the marked work. Problems? If you are having study related or technical problems with this document, make sure you contact your assessor at the earliest convenience to get the problem resolved. The name of your assessor is located on Page 1, and the contact details can be found at; www.cffet.net/env/contacts Resources & references References Sturman, A.P, Tapper, N.J., (2000). The weather and climate of Australia and New Zealand. Oxford University Press. Melbourne. Australia. The Shodor Education Foundation Inc. Air Quality Meteorology. A Developmental Course of the US Environmental Protection Agency in conjunction with the US National Oceanic and Atmospheric Administration. http://www.shodor.org/metweb/index.html Bureau of Meteorology. Melbourne. Department http://www.bom.gov.au (accessed 18/04/08) of Environment & Heritage Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 20 Collect & evaluate meteorological data Woodruff, S.W. Atmospheric Stability. Pierce http://www.piercecollege.edu/offices/weather/stability.html (accessed 2007-8) Study Module 5 College. further reading & online learning aids Further reading & online aids MetExplore spreadsheet (see teacher) contains interactive information on equations of state, gas laws, pressure/height relationships, vapor pressure and other related material. Hunter TAFE - Chemical, Forensic, Food & Environmental Technology [cffet.net] Course Notes for delivery of MSS11 Sustainability Training Package Page | 21