Which NICS Aromaticity Index for Planar π Rings Is Best for the
advertisement

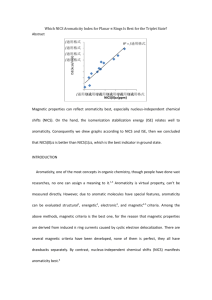
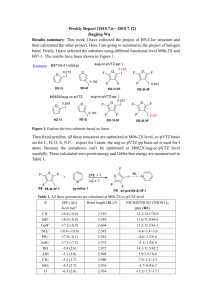
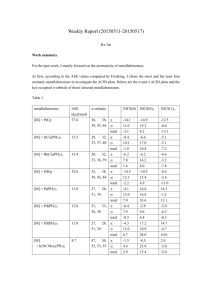
Which NICS Aromaticity Index for Planar π Rings Is Best for the Triplet State? Hongchao Sun, Ke An, Jun Zhu* State Key Laboratory of Physical Chemistry Solid Surface and Fujian Provincial Key Laboratory of Theoretical and Computational Chemistry, College of Chemistry and Chemical Engineering, Xiamen University , Xiamen, Fujian 361005, China Abstract Aromaticity is always a hot issue, since NICS have gained popularity among theoretical chemists to judge aromaticity, many reports on aromaticity have been delivered and many others will be published, too. On the other hand, the isomerization stabilization energies (ISE) relate are used to measure aromaticity. Combining the two methods together, during our research, we found that they had good relationships, we drew graphs according to ISE vs NICS, then we concluded that NICS(1)zz is better than NICS (0) zz, it was consistent with previous researches. We provided a inexpensive and convenient method to evaluate aromaticity. INTRODUCTION Aromaticity, one of the most important concepts in organic chemistry, though people have done vast researches, no one can assign an exact meaning to it.1,2 Aromaticity (1) (2) Krygowski, T. M.; Cyrański, M. K. Chemical Reviews 2001, 101, 1385. Gomes, J. A. N. F.; Mallion, R. B. Chemical Reviews 2001, 101, 1349. is virtual property, can’t be measured directly. However, due to aromatic molecules have special features, it can be evaluated through structural,1 electronic,2energetic,3 magnetic 4 and reactivity 5 criterion. Before nucleus-independent chemical shifts (NICS) were proposed, there were several magnetic criteria had been developed, such as exalted magnetic susceptibilities(Λ),6 7Li+ NMR chemical shifts,7,8 3He NMR chemical shifts.9 Besides there is another well-known criterion, Hückel 4n+2 rule,10,11 a cyclic(planar) system containing 4n+2 π-electron is aromatic, on the contrary if it has 4n π-electron then it is antiaromaticity. This criterion is efficient in the ground state and has been widely accepted, chemists reformed it and applied to other systems, for example, the discovery of Wilson12 confirmed that Hückel’s role could be used to assess the stability of chelate compounds, Winstein13 explained the generation of homoaromaticity through it, Breslow 14 identified that cyclopropenyl anions were antiaromatic. In 1972, Baird15 found that “ 4n rings are aromatic and 4n+2 rings antiaromatic in such triplets”, such triplets meant the lowest 3ππ* state. This new (3) Cyrański, M. K. Chemical Reviews 2005, 105, 3773. (4) Chen, Z.; Wannere, C. S.; Corminboeuf, C.; Puchta, R.; Schleyer, P. v. R. Chemical Reviews 2005, 105, 3842. (5) Krygowski, T. M.; Cyrañski, M. K.; Czarnocki, Z.; Häfelinger, G.; Katritzky, A. R. Tetrahedron 2000, 56, 1783. (6) Dauben, H. J., Jr.; Wilson, J. D.; Laity, J. L. J. Amer. Chem. Soc. 1968, 90, 811. (7) Paquette, L. A.; Bauer, W.; Sivik, M. R.; Buehl, M.; Feigel, M.; Schleyer, P. v. R. Journal of the American Chemical Society 1990, 112, 8776. (8) Buehl, M.; Thiel, W.; Jiao, H.; Schleyer, P. v. R.; Saunders, M.; Anet, F. A. L. Journal of the American Chemical Society 1994, 116, 6005. (9) Saunders, M.; Jimenez-Vazquez, H. A.; Cross, R. J.; Billups, W. E.; Gesenberg, C.; Gonzalez, A.; Luo, W.; Haddon, R. C.; Diederich, F.; Herrmann, A. Journal of the American Chemical Society 1995, 117, 9305. (10) Huckel, E. Z. Phys. 1931, 70, 204. (11) Huckel, E. Z. Elektrochem. Angew. Phys. Chem. 1937, 43, 752. (12) Calvin, M.; Wilson, K. W. Journal of the American Chemical Society 1945, 67, 2003. (13) Winstein, S. Journal of the American Chemical Society 1959, 81, 6524. (14) Breslow, R.; Brown, J.; Gajewski, J. J. Journal of the American Chemical Society 1967, 89, 4383. (15) Baird, N. C. Journal of the American Chemical Society 1972, 94, 4941. criterion is called Baird’s rule, which is contrary to Hückel’s role. Also Dewar, 16 Schleyer17,18,19 and other chemists enriched and consummate Hückel’s role. None of them is perfect, they all have drawbacks separately. By contrast, NICS is considered to be a more successful aromaticity index when it was applied to evaluate organic compounds than other magnetic criteria.4 METHDOLOGY All molecular geometries were optimized using Density Functional Theory at B3LYP/6-311++G(d,p) level, the ISE values were pure electronic energies without correction, NICS were also calculated at B3LYP/6-311++G(d,p) level. We selected three points to calculate NICS, at ring centers and 1 Å away from centers perpendicularly, above and below. However, Grey 20 reported that curvature of molecular surfaces could influence NICS remarkably, moreover, concave surfaces had greater effects than convex surfaces on chemical shielding. One of the features of a aromaticity compound is that it has a planar or spherical structure, therefore we selected planar molecules in triplet state to continue our research. In this way, NICS(1)zz and NICS(-1)zz had equal values, and the same to NICS(1)iso and NICS(-1)iso. Generally negative NICS values indicate induced ring currents and aromaticity while positive values mean paratropic ring currents and antiaromaticity.21 (16) Dewar, M. J. S. Bulletin des Sociétés Chimiques Belges 1979, 88, 957. (17) Jemmis, E. D.; Schleyer, P. v. R. Journal of the American Chemical Society 1982, 104, 4781. (18) Fokin, A. A.; Jiao, H.; Schleyer, P. v. R. Journal of the American Chemical Society 1998, 120, 9364. (19) Wannere, C. S.; Corminboeuf, C.; Wang, Z.-X.; Wodrich, M. D.; King, R. B.; Schleyer, P. v. R. Journal of the American Chemical Society 2005, 127, 5701. (20) Forse, A. C.; Griffin, J. M.; Presser, V.; Gogotsi, Y.; Grey, C. P. The Journal of Physical Chemistry C 2014, 118, 7508. (21) von Ragué Schleyer, P.; Manoharan, M.; Wang, Z.-X.; Kiran, B.; Jiao, H.; Puchta, R.; van Eikema Hommes, N. J. R. Organic Letters 2001, 3, 2465. Judged by it, NICS(1)zz and NICS(1)iso coordinated better with ISE values than the other three indices. RESULTS AND DISSCUSSION In 1996, NICS were proposed by Schleyer,22 as it could evaluate aromaticity and antiaromaticity of wide range molecules, also it didn’t need references, what’s more, they correlated well with other criteria. Togethering with Schleyer, Heine23 reported that NICSzz was a good measure for [n]-annulenes, especially NICS(1)πzz and NICS(1)zz. Carpenetti24 used NICS(1)zz to measure the antiaromaticity of indenyl and fluorenyl cationic systems, the conclusion drew by this method was consistent with the results of 1H NMR shifts. Laali25 proved that NICS(1)zz was also a more reliable probe than NICS(1) when computed in janusenes. In 2006, Schleyer26 reported that NICS (0)πzz were the best and most reliable aromaticity indices among their selected NICS indices, besides they were linear with aromatic stabilization energies (ASE), NICS(1)zz had good correlations, too. Furthermore, Mills 27 justified that NICS(1)zz were accurate reflections of local aromaticity, this conclusion was sustained by the excellent linear relationship with magnetic susceptibility exaltation and indirectly validated by the excellent correlation between experimental shifts and 13C NMR (22) Schleyer, P. v. R.; Maerker, C.; Dransfeld, A.; Jiao, H.; Hommes, N. J. R. v. E. Journal of the American Chemical Society 1996, 118, 6317. (23) Corminboeuf, C.; Heine, T.; Seifert, G.; Schleyer, P. v. R.; Weber, J. Phys. Chem. Chem. Phys. 2004, 6, 273. (24) Mills, N. S.; Llagostera, K. B.; Tirla, C.; Gordon, S. M.; Carpenetti, D. J Org Chem 2006, 71, 7940. (25) Okazaki, T.; Laali, K. K. Org. Biomol. Chem. 2006, 4, 3085. (26) Fallah-Bagher-Shaidaei, H.; Wannere, C. S.; Corminboeuf, C.; Puchta, R.; Schleyer, P. v. R. Organic Letters 2006, 8, 863. (27) Mills, N. S.; Llagostera, K. B. J. Org. Chem. 2007, 72, 9163. shifts calculated density functional theory (DFT), B3LYP/6-311+g(d, p). Palusiak28 verified that NICS(1)zz of phenylic rings had the largest linear regression versus total electron energies among NICS(0),NICS(1) and NICS(1)zz, they might be served as a standard to measure whether a molecule is aromaticity or not. Ebrahimi 29 used NICS(1)zz to detect the ring aromaticity changes on complexation, it was supported by the excellent correlation of the electron density changes at the center of ring against the changes of NICS(1)zz. While NICS are widely used as an evaluation of aromaticity or antiaromaticity for molecules in ground state, few reports on the aromaticity of excited molecules recognized in this way have been delivered. So, which NICS indices can manifest aromaticity best among these easily computed magnetism-based tensors? Last year, our team 30 reported a method that methyl-methylene isomerization stabilization energies (ISEI), which was applied to evaluate aromaticity by Pühlhofer31 in 2002, used to identify the aromaticity of selected nine species, correlated well with NICS (1)zz in T1 state. Additionally we adopted another method, indene-isoindene isomerization stabilization energies (ISEII) to investigate the aromaticity of [4n]annulenes, these values were still correlated well with NICS (1)zz in T1 state.32 However, we didn’t describe other NICS indices’ performance. Herein, we will make a supplement (Table 1), including four NICS tensors and all of the ISE values calculated through the two different methods mentioned above. (28) (29) (30) (31) (32) Palusiak, M.; Krygowski, T. M. Chem. - Eur. J. 2007, 13, 7996. Ebrahimi, A.; Habibi, M.; Masoodi, H. R.; Gholipour, A. R. Chem. Phys. 2009, 355, 67. Zhu, J.; An, K.; Schleyer, P. v. R. Organic Letters 2013, 15, 2442. Schleyer, P. v. R.; Pühlhofer, F. Organic Letters 2002, 4, 2873. An, K.; Zhu, J. Eur. J. Org. Chem. 014, 2764.2014, 2. Table 1. ISE (kcal/mol) and NICS aromaticity indices (in ppm) at the ring centers and 1 Å above for monocyclic compounds in the T 1 state at the B3LYP/6-311+G** level. Entry Species NICS(1)zz NICS(0)zz NICS(1)iso NICS(0)iso ISEI ISEII 1 66.9 100.9 21.8 30 16.9 15.5 2 -21.1 -4.1 -8.8 -1.4 -14.5 -14.1 3 -32.7 -26.1 -10 -10.6 -24.6 -19.3 4 -32.4 -29.3 -10.9 -11.1 -24.7 -17.0 5 2.3 28.4 0.1 3.2 0.6 -2.1 6 -25.1 -3.2 -10.3 -2.5 -22.5 -20.9 7 -20.2 -3.1 -8.6 -3.7 -13.7 -17.1 8 -20.3 0.3 -7.7 0.2 -16.8 -20.1 9 -17.2 22.8 -6.8 -2.1 -16.4 -16.2 The relationships of ISEI and ISEII against NICS indices were shown in Figure 1 and Figure 2 separately. For ISEI method (Figure 1), apparently NICS (1) zz was the best aromaticity index for these monocyclic species with 4n π-electrons. Meanwhile, as for ISEII (Figure 2), the performance of NICS (1) iso (r2 = 0.940) was better than NICS (1) zz (r2 = 0.930), but they had a very narrow difference, and NICS (1) iso were more subject to environment, actually we could still considered NICS (1) zz as the best index among them. Figure 1. Plots of ISEI vs four NICS indices [NICS(1)zz, NICS(0)zz, NICS(1)iso, NICS(0)iso] in table 1. Figure 2. Plots of ISEII vs four NICS indices [NICS(1)zz, NICS(0)zz, NICS(1)iso, NICS(0)iso] in table 1. As previous works23-29 were focused on [4n]annulenes, we weren’t aware whether NICS and ISEI were applicable to the evaluate aromaticity of five-membered heterocycles in T1 or not. Now we report it here. Restricted by the definition of NICS, all the compounds we chosen had planar structures in triplet state. Under such conditions, we couldn’t separate π contribution to NICS tensor through canonical molecular orbital (CMO) NICS method, as a result, NICSπ indices weren’t taken into consideration. The homodesmotic reaction (Scheme S1, Supporting Information) was used to calculate the ISEI values in Table 2. Table 2. ISEI (kcal/mol) and NICS for five-membered planar heterocycles in the T 1 state. Entry compound NICS(1)zz NICS(0)zz NICS(1)iso NICS(0)iso ISEI 10 -12.5 4.6 -3.2 5.5 -7.6 11 -12.5 0.8 -5.2 -0.4 -10.9 12 -14.6 5.3 -5.4 1.0 -10.2 13 -9.1 12.7 -4.7 -1.9 -4.3 14 -3.4 18.8 -2.3 1.4 -1.7 15 -4.3 15.8 -2.6 1.5 -3.9 16 -7.2 14.3 -4.5 -1.9 -3.2 17 -6.6 16.3 -4.2 -2.1 -1.0 18 -5.7 7.1 -2.0 -2.0 -4.9 19 10.7 38.5 1.6 7.9 1.3 20 10.6 32.3 2.9 3.2 2.1 From species 10 to 18, they had 4 π electrons, species 19 and 20 had 6 π electrons, according to Baird’s rule, they were aromatic, indeed their NICS (1) zz and ISEI values were negative, generally considered as a criterion judging molecules’ aromaticity. However, in Figure 3, the statistical correlations were different from previous performance, NICS (0) zz manifested best, such a result was contradictory with other researches, then we combined present data with former data derived from ref. 30 (Table S1, Supporting Information). The correlations ISEI against the other NICS indices (Figure 4) were different from those in Figure 3, NICS (1) NICS (0) zz decreased, and NICS (1) iso and NICS (0) iso zz increased while were also enhanced. But, statistically, there was one aspect consistent with other reports, compared with other indices NICS (1) zz correlated with ISEI best. Figure 3. Plots of ISEI vs four NICS indices [NICS(1)zz, NICS(0)zz, NICS(1)iso, NICS(0)iso] in table 2 Figure 4. Plots of total ISEI vs four NICS indices [NICS(1)zz, NICS(0)zz, NICS(1)iso, NICS(0)iso] in table 3 CONCLUSION In this text, the good relationship between ISEI and NICS(1)zz values indicated that NICS(1)zz can act as a criterion to measure the aromaticity of five-membered rings. Such a method has an advantage, easily to be calculated.