Group B-A5 REPOST - critical chemistry
advertisement

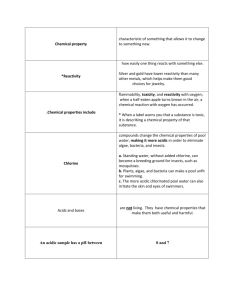
Acids and Bases (Summary…) This text (chapter 15) refers to acids and bases. According to this text, the basic and most common definition for acids and bases was defined by Bronsted and Lawry and is the following: Acid is called any substance which donates a proton, for example NH4+. Base is called any substance which accepts a proton, just like NH3. A conjugate acid-base pair is called a pair of a strong acid and its conjugate (weak) base, when the acid has lost a proton or a strong base and its conjugate (weak) acid, respectively. To continue with, a substance is called amfoteric, like water, when k w= [H3O+] = [OH-]. The acidity of an aqueous solution is measured by a specific scale which is called pH and it is defined as the negative logarithm of the hydrogen ion concentration (M): pH= -log [H+]. On the other side, the basisity of an aqueous solution is measured by the definition of pOH, which is defined as the negative logarithm of the hydroxide ion concentration (M): pOH= -log [OH-]. From these definitions we can observe that pH+ pOH= 14. Furthermore an acid solution has pH<7, a basic solution has pH>7 and a neutral one has pH=7. Acids and bases can be either strong or weak. The similarities in strength of acids can be explained qualitatively in terms of their molecular structures. The first ones have the ability to ionize completely in water, like HCl and HNO3 from the acids and NaOH, Ba(OH)2 from the bases, in contrast to the second ones which ionize partially in water, such as HF and CH3COOH from the acids and NH3 from the bases. Strong bases include hydroxides of alkali metals and of alkaline earth metals. Every acid and base has an equilibrium constant for its ionization. Any acid has its unique ionization constant Ka and any base has its own ionization constant Kb. These ionization constants express the strength of the acids and bases respectively. The acid ionization constant increases as the acid strength grows and in the same way for the bases, Kb increases with the base strength. We can observe that for an acid and its conjugate base or the opposite, it applies: Ka× Kb= Kw. Percent ionization (α) is another measure of the strength of acids and bases and it is defined, dividing the concentration of the ionized acid to the initial concentration of the acid × 100%. The more dilute a solution of a weak acid is, the greater the percent ionization of the acid is. Ionic compounds that are formed by the reaction between an acid and a base are called salts. The reaction of these compounds with water is called salt hydrolysis and can produce acidic or basic solutions. The salts are either strong electrolytes, when they are derived from neutral solutions, or basic salts, when they are derived from weak acid-strong base, or acidic salts, when they come from strong acid-weak base. There is also another type of salt which is formed by weak acid-weak base and it is basic when: Ka<Kb, acidic when: Ka>Kb and neutral when: Ka≈ Kb. The last part of the text given refers to the acids and bases as they were defined by Lewis. A Lewis acid accepts a pair of electrons, like BF3 and it doesn’t contain necessary ionized hydrogen atoms. A Lewis base on the other hand donates a pair of electrons, like NH3.