NPH_4190_sm_NotesS2
advertisement

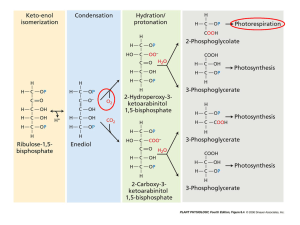
Supporting Information Notes S2 Possibility of decreasing the low-molecular-mass water-soluble P esters A significant economy in the use of P by plants could be achieved by decreasing the content of low-Mr (relative molecular mass) water-soluble phosphate esters, which represent approximately 15–20% of total P in most plants (main text Fig. 1, inset). How could a decrease in the low-Mr water-soluble ester P be achieved? One possibility is to alter metabolic pathways radically by genetic modification of plants to decrease the total number of phosphorylated water-soluble intermediates, with emphasis on eliminating reactions involving a low-Mr water-soluble P ester present at a high concentration. A second possibility is to retain the existing pathways of metabolism and to alter one or more enzymes in a way which, as an emergent property, involves lower concentrations of a low-Mr water-soluble P ester. A third possibility is the addition of reactions to a pre-existing pathway which decreases the overall use of P. Most of the suggestions of genetic modification of plants that involve pathways with phosphorylated intermediates concern photosynthetic CO2 assimilation. The most radical possibility is that of altering metabolic pathways by genetic modification of plants to decrease the total number of phosphorylated water-soluble intermediates, with emphasis on eliminating reactions involving a low-Mr water-soluble P ester present at a high concentration. Perhaps the best way to address this issue is to determine what the plants themselves are capable of. Under severe Pi stress, several enzymatic bypasses for steps in glycolysis that require P-esters or adenylates are upregulated (see; Plaxton & Podestá, 2006; Plaxton & Tran, 2011). These bypasses do not require Pi or adenylates, allowing respiratory C- flux through glycolysis to continue to generate energy and to produce the C-skeletons needed for key anabolic processes under conditions where available Pi and/or adenylate charge are decreased. Some bypass enzymes such as pyrophosphate (PPi)-dependent phosphofructokinase and pyruvate orthophosphate dikinase use PPi to accomplish cellular work. Another enzyme that uses PPi, a tonoplast H+-PPiase, is also induced under Pi deficiency, helping to maintain vacuolar pH at lower adenylate charge (Palma et al., 2000). These and other enzymatic bypasses may provide opportunities for reducing the amount of Pi locked up in P-ester pools. The suggestion by Bar-Even et al. (2010) of replacing ribulose bisphosphate carboxylaseoxygenase (Rubisco) and the associated photosynthetic carbon reduction cycle (PCRC) by another autotrophic CO2 assimilation pathway was not aimed at economising on low-Mr watersoluble P esters, but rather at eliminating the oxygenase activity of Rubisco with its associated energy costs and also a decreased energy cost for CO2 assimilation while decreasing the halfsaturation value for CO2 in photosynthesis. Bar-Even et al. (2010) selected reactions from the five known autotrophic CO2 fixation pathway other than Rubisco-PCRC which are not inhibited by O2 and (for carboxylases) have a high CO2 affinity; from the present viewpoint the important feature is that the five other pathways have many fewer phosphorylated intermediates than Rubisco-PCRC (see: Raven, 2009; Bar-Even et al., 2010; Berg, 2011). Since the PCRC or the oxidative pentose phosphate pathway are the only source of erythrose-4-P used, with phosphoenolpyruvate, in the shikimate pathway of aromatic synthesis, some of enzymes of the pentose cycles and their phosphorylated intermediates must be present even if the reductant generating role of the oxidative pentose phosphate pathway is replaced by NADP+ reduction by, for example, cytosolic NADP+-linked malate dehydrogenase or isocitrate dehydrogenase. Since the five pathways of autotrophic CO2 assmilation other than Rubisco – PCRC all require the glucogenetic pathway to produce sugars and 3-phosphoglycerate, the only phosphorylated compounds associated with Rubisco –PCRC and the photorespiratory carbon oxidation cycle, but not the other five pathways or the oxidative pentose phosphate pathway are ribulose-1.5bisphosphate (RuBP) sedoheptulose-1.7-bisphosphate (SBP) and 2-phosphoglycolate. The SBP and 2-phosphoglycolate concentrations are generally low, while RuBP is usually a, if not the, major component of the stromal pool of low-Mr water-soluble P ester with a significant fraction bounds to Rubisco and associated with Mg2+ (von Caemmerer & Edmondson, 1986; Gerhardt et al., 1987; Servaites & Geiger, 1995; Ruuska et al., 2000; Viil et al., 2001). A much smaller fraction of 3-phosphoglycerate is bound to Rubisco, where it acts as a negative effector (von Caemmerer & Edmondson, 1986; Woodrow & Berry, 1988; Servaites & Geiger, 1995). Rubiscosaturating concentrations of RuBP are essential for maintaining Rubisco activation (Woodrow & Berry, 1988; Servaites & Geiger, 1995) and a high CO2 affinity. It is unlikely that the replacements of the Rubisco – PCRC with a pathway having fewer low-Mr water-soluble P ester intermediates would only decrease the content of the esters by 10% at most. Whether such wholesale intervention as replacing Rubisco - PCRC is justified by the P saving needs further investigation, since using the data in Table 1 with total plant P equal to 8.4 times the content of low-Mr water-soluble ester P, decreasing the content of these P esters by 10% would decrease the whole plant P content by not more than 1.2%. A second possibility is to retain the existing pathways of metabolism and to alter one of more enzymes in a way which, as an emergent property, involves lower concentrations of (a) low-Mr water-soluble P ester(s). Whitney et al. (2011) survey achievements and opportunities in replacing the native Form IB Rubisco in a C3 plant with a Rubisco (e.g. a Form ID Rubisco) with different kinetic properties (Whitney et al., 2011). Whether this would economise on P through a decreased number of Rubisco active sites binding RuBP for a given rate of CO2 assimilation requires further investigation. It seems unlikely that this intervention could yield as large a decrease in whole-plant P as the 1.2% for the first mechanism. A third possibility is the addition of reactions to a pre-existing pathway which decreases the overall use of P. The possibilities here include the addition of respiratory bypass enzymes to eliminate Pi requiring steps (Plaxton & Podestá, 2006; Plaxton & Tran, 2011), a CCM based on C4 metabolism (Kajala et al., 2011; Miyao et al., 2011) or of a cyanobacterial CCM (Price et al., 2011) to C3 crop plants. Addition of CCMs would permit the Form IB Rubisco with high CO2 affinity, high CO2/O2 selectivity but low CO2-saturated specific reaction rate with a Rubisco with lower CO2 affinity and low CO2/O2 selectivity but higher CO2-saturated specific reaction rate. Such a Rubisco, supplied with saturating CO2 by the CCM, could be present as fewer copies while retaining, or increasing, the photosynthetic rate. Such a replacement of Rubisco could involve lower RuBP concentrations with less RuBP bound to Rubisco, consistent with data showing higher phosphorus use efficiency in two C4 grasses than in a C3 grass (Ghannoum & Conroy, 2007; Ghannoum et al., 2008). Further investigation is needed, especially, in the case of a C4 CCM, of any trade-off in C4 plants between decreased RuBP requirement and a possible increased phosphoenolpyruvate steady state concentration to serve the photosynthetic role of phosphoenolpyruvate carboxylase and phosphoenolpyruvate carboxykinase than is needed for the glycolytic and anaplerotic involvement of phosphoenolpyruvate (Aubry et al., 2011). As with the second mechanism, it is very unlikely that a whole-plant P saving could be achieved that is as great as the maximum of 1.2% possible with the first mechanism discussed above, To summarise, genetic modification of pathways could only yield small decreases in overall plant P requirement by decreasing the concentration of low-Mr water-soluble P esters. References Aubry S, Brown NJ, Hibberd JM. 2011. The role of proteins in C3 plants prior to their recruitment into the C4 pathway. Journal of Experimental Botany 62(9): 3049-3059. Bar-Even A, Noor E, Lewis NE, Milo R. 2010. Design and analysis of synthetic carbon fixation pathways. Proceedings of the National Academy of Sciences of the United States of America 107(19): 88898894. Berg IA. 2011. Ecological aspects of the distribution of different autotrophic CO2 fixation pathways. Applied and Environmental Microbiology 77(6): 1925-1936. Gerhardt R, Stitt M, Heldt HW. 1987. Subcellular metabolite levels in spinach leaves - regulation of sucrose synthesis during diurnal alterations in photosynthetic partitioning. Plant Physiology 83(2): 399-407. Ghannoum O, Conroy JP. 2007. Phosphorus deficiency inhibits growth in parallel with photosynthesis in a C3 (Panicum laxum) but not two C4 (P. coloratum and Cenchrus ciliaris) grasses. Functional Plant Biology 34(1): 72-81. Ghannoum O, Paul MJ, Ward JL, Beale MH, Corol DI, Conroy JP. 2008. The sensitivity of photosynthesis to phosphorus deficiency differs between C3 and C4 tropical grasses. Functional Plant Biology 35(3): 213-221. Kajala K, Covshoff S, Karki S, Woodfield H, Tolley BJ, Dionora MJA, Mogul RT, Mabilangan AE, Danila FR, Hibberd JM, Quick WP. 2011. Strategies for engineering a two-celled C4 photosynthetic pathway into rice. Journal of Experimental Botany 62(9): 3001-3010. Miyao M, Masumoto C, Miyazawa SI, Fukayama H. 2011. Lessons from engineering a single-cell C4 photosynthetic pathway into rice. Journal of Experimental Botany 62(9): 3021-3029. Palma DA, Blumwald E, Plaxton WC. 2000. Upregulation of vacuolar H+-translocating pyrophosphatase by phosphate starvation of Brassica napus (rapeseed) suspension cell cultures. Febs Letters 486: 155-158. Plaxton WC, Podestá FE. 2006. The functional organization and control of plant respiration. Critical Reviews in Plant Science 25: 159-198. Plaxton WC, Tran HT. 2011. Metabolic adaptations of phosphate-starved plants. Plant Physiology 156: 1006-1015. Price GD, Badger MR, von Caemmerer S. 2011. The prospect of using cyanobacterial bicarbonate transporters to improve leaf photosynthesis in C3 crop plants. Plant Physiology 155(1): 20-26. Raven JA. 2009. Contributions of anoxygenic and oxygenic phototrophy and chemolithotrophy to carbon and oxygen fluxes in aquatic environments. Aquatic Microbial Ecology 56(2-3): 177-192. Ruuska SA, Andrews TJ, Badger MR, Price GD, von Caemmerer S. 2000. The role of chloroplast electron transport and metabolites in modulating Rubisco activity in tobacco. Insights from transgenic plants with reduced amounts of cytochrome b/f complex or glyceraldehyde 3-phosphate dehydrogenase. Plant Physiology 122(2): 491-504. Servaites JC, Geiger DR. 1995. Regulation of ribulose 1,5-bisphosphate carboxylase oxygenase by metabolites. Journal of Experimental Botany 46: 1277-1283. Viil Y, Ivanova H, Parnik T, Parsim E. 2001. Measurement of the concentration of the active centers of ribulose-1,5-bisphosphate carboxylase/oxygenase in vivo. Russian Journal of Plant Physiology 48(1): 116-121. von Caemmerer S, Edmondson DL. 1986. Relationship between steady-state gas-exchange, invivo ribulose bisphosphate carboxylase activity and some carbon-reduction cycle intermediates in Raphanus sativus. Australian Journal of Plant Physiology 13(5): 669-688. Whitney SM, Houtz RL, Alonso H. 2011. Advancing our understanding and capacity to engineer nature's CO2-sequestering enzyme, Rubisco. Plant Physiology 155(1): 27-35. Woodrow IE, Berry JA. 1988. Enzymatic regulation of photosynthetic CO2 fixation in C3 plants. Annual Review of Plant Physiology and Plant Molecular Biology 39: 533-594.