Solutions Unit Test Outline
advertisement

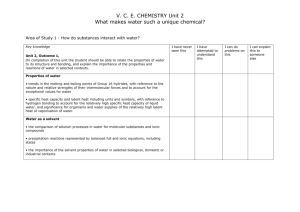
SCH 3U Name_________________________ Solution Unit Test Review Outline I can define key terms relating to solutions: Solute, solvent, concentrated, dilute, electrolyte, nonelectrolyte, miscible, immiscible, standard solution, saturated, unsaturated, supersaturated, crystallization, qualitative and quantitative analysis, ionization, dissociation, titration, titrant, equivalence point, endpoint, analyte I can describe the characteristics of a solution I can explain (with diagrams) how molecular and ionic compounds dissolve in water I can explain how surfactants and emulsifiers work and give examples of these types of substances I can calculate amount concentration using 𝑐 = 𝑛 𝑉 I can perform calculations related to dilutions using 𝑐1 𝑉1 = 𝑐2 𝑉2 I can perform percent concentration calculations used in consumer products (w/w, v/v, w/v) and adjust units accordingly, using 𝑐 = 𝑠𝑜𝑙𝑢𝑡𝑒 𝑠𝑜𝑙𝑢𝑡𝑖𝑜𝑛 × 100 I can calculate very small concentrations in units of ppm or ppb using 𝑐 = or 𝑐 = 𝑠𝑜𝑙𝑢𝑡𝑒 𝑠𝑜𝑙𝑢𝑡𝑖𝑜𝑛 𝑠𝑜𝑙𝑢𝑡𝑒 𝑠𝑜𝑙𝑢𝑡𝑖𝑜𝑛 × 106 × 109 I can explain how changes in temperature and pressure affect the solubility of solids, liquids, and gases; I can interpret the information on a solubility curve I can represent reaction in solution using total and net ionic equations I can use the solubility table to determine whether a reaction in solution will produce a precipitate I can design a sequential chemical analysis, using the solubility table, to test an unknown solution for multiple ions I can use stoichiometric calculations to make predictions about quantities in solution reactions I can describe properties of acids and bases I can describe Arrhenius and Bronsted-Lowry definitions of acids and bases I can identify conjugate acids and bases I can calculate pH given [H+] and I can calculate [H+] given pH I can perform stoichiometric calculations relating to acid-base neutralizations (titration) I can describe application of neutralization reactions in the human body I can explain the difference between a strong acid and a weak acid (give examples of each) Practice Questions: Pg 500-501 # 3-9, 11-18; Pg. 502-505 # 4, 10-12, 16, 17, 19, 22, 25, 26, 28, 43, 44, 46- 50, 60, 75 SCH 3U Name_________________________ Solution Unit Test Review Outline I can define key terms relating to solutions: Solute, solvent, concentrated, dilute, electrolyte, nonelectrolyte, miscible, immiscible, standard solution, saturated, unsaturated, supersaturated, crystallization, qualitative and quantitative analysis, ionization, dissociation, titration, titrant, equivalence point, endpoint, analyte I can describe the characteristics of a solution I can explain (with diagrams) how molecular and ionic compounds dissolve in water I can explain how surfactants and emulsifiers work and give examples of these types of substances I can calculate amount concentration using 𝑐 = 𝑛 𝑉 I can perform calculations related to dilutions using 𝑐1 𝑉1 = 𝑐2 𝑉2 I can perform percent concentration calculations used in consumer products (w/w, v/v, w/v) and adjust units accordingly, using 𝑐 = 𝑠𝑜𝑙𝑢𝑡𝑒 𝑠𝑜𝑙𝑢𝑡𝑖𝑜𝑛 × 100 I can calculate very small concentrations in units of ppm or ppb using 𝑐 = or 𝑐 = 𝑠𝑜𝑙𝑢𝑡𝑒 𝑠𝑜𝑙𝑢𝑡𝑖𝑜𝑛 𝑠𝑜𝑙𝑢𝑡𝑒 𝑠𝑜𝑙𝑢𝑡𝑖𝑜𝑛 × 106 × 109 I can explain how changes in temperature and pressure affect the solubility of solids, liquids, and gases; I can interpret the information on a solubility curve I can represent reaction in solution using total and net ionic equations I can use the solubility table to determine whether a reaction in solution will produce a precipitate I can design a sequential chemical analysis, using the solubility table, to test an unknown solution for multiple ions I can use stoichiometric calculations to make predictions about quantities in solution reactions I can describe properties of acids and bases I can describe Arrhenius and Bronsted-Lowry definitions of acids and bases I can identify conjugate acids and bases I can calculate pH given [H+] and I can calculate [H+] given pH I can perform stoichiometric calculations relating to acid-base neutralizations (titration) I can describe application of neutralization reactions in the human body I can explain the difference between a strong acid and a weak acid (give examples of each) Practice Questions: Pg 500-501 # 3-9, 11-18; Pg. 502-505 # 4, 10-12, 16, 17, 19, 22, 25, 26, 28, 43, 44, 46- 50, 60, 75