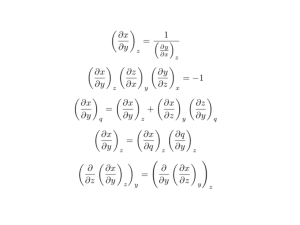eprint_12_15153_5018
advertisement

CERAMIC PHASE DIAGRAMS It need not be assumed that phase diagrams exist only for metal–metal systems; in fact, phase diagrams that are very useful in the design and processing of ceramic systems have been experimentally determined for quite a number of these materials. For binary or two-component phase diagrams, it is frequently the case that the two components are compounds that share a common element, often oxygen. These diagrams may have configurations similar to metal–metal systems, and they are interpreted in the same way. THE Al2O3–Cr2O3 SYSTEM One of the relatively simple ceramic phase diagrams is that found for the aluminum oxide– chromium oxide system, Figure 1. This diagram has the same form as the isomorphous copper–nickel phase diagram consisting of single liquid and single solid phase regions separated by a two-phase solid–liquid region having the shape of a blade. The Al2O3–Cr2O3 solid solution is a substitutional one in which Al3_ substitutes for Cr3_, and vice versa. It exists for all compositions below the melting point of Al2O3 inasmuch as both aluminum and chromium ions have the same charge as well as similar radii (0.053 and 0.062 nm, respectively). Furthermore, both Al2O3 and Cr2O3 have the same crystal structure. FIGURE 1 The aluminum oxide–chromium oxide THE MgO–Al2O3 SYSTEM The phase diagram for the magnesium oxide–aluminum oxide system (Figure 2) is similar in many respects to the lead–magnesium diagram . There exists an intermediate phase, or better, a compound called spinel, which has the chemical formula MgAl2O4 (or MgO– Al2O3). Even though spinel is a distinct compound [of composition 50 mol% Al2O3–50 mol% MgO (72 wt% Al2O3–28 wt% MgO)], it is represented on the phase diagram as a singlephase field rather than as a vertical line, as for Mg2Pb (Figure 10.18); that is, there is a range of compositions over which spinel is a stable compound. Thus, spinel is nonstoichiometric for other than the 50 mol% Al2O3–50 mol% MgO composition. Furthermore, there is limited solubility of Al2O3 in MgO below about 1400_C (2550_F) at the left-hand extremity of Figure 10.22, which is due primarily to the differences in charge and radii of the Mg2_ and Al3_ ions (0.072 versus 0.053 nm). For the same reasons, MgO is virtually insoluble in Al2O3 , as evidenced by a lack of a terminal solid solution on the righthand side of the phase diagram. Also, two eutectics are found, one on either side of the spinel phase field, and stoichiometric spinel melts congruently at about 2100_C (3800_F). The magnesium oxide–aluminum oxide phase diagram. 2FIGURE THE ZrO2–CaO SYSTEM Another important binary ceramic system is that for zirconium oxide (zirconia) and calcium oxide (calcia); a portion of this phase diagram is shown in Figure 3. The horizontal axis extends to only about 31 wt% CaO (50 mol% CaO), at which composition the compound CaZrO3 forms. It is worth noting that one eutectic (2250_C and 23 wt% CaO) and two eutectoid (1000_C and 2.5 wt% CaO, and 850_C and 7.5 wt% CaO) reactions are found for this system. It may also be observed from Figure 10.23 that ZrO2 phases having three different crystal structures exist in this system, namely tetragonal, monoclinic, and cubic. Pure ZrO2 experiences a tetragonal-to-monoclinic phase transformation at about 1150_C (2102_F). A relatively large volume change accompanies this transformation, resulting in the formation of cracks that render a ceramic ware useless. This problem is overcome by ‘‘stabilizing’’ the zirconia by adding between about 3 and 7 wt% CaO. Over this composition range and at temperatures above about 1000_C both cubic and tetragonal phases will be present. Upon cooling to room temperature under normal cooling conditions, the monoclinic and CaZr4O9 phases do not form (as predicted from the phase diagram); consequently, the cubic and tetragonal phases are retained, and crack formation is circumvented. A zirconia material having a calcia content within the range cited above is termed a partially stabilized zirconia, or PSZ. Yttrium oxide (Y2O3) and magnesium oxide are also used as stabilizing agents. Furthermore, for higher stabilizer contents, only the cubic phase may be retained at room temperature; such a material is fully stabilized. FIGURE 3 A portion of the zirconia–calcia phase diagram .