Blood
advertisement

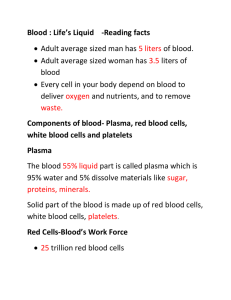
THE BLOOD Functions of Blood Blood has three general functions: 1. Transportation. blood transports oxygen from the lungs to the cells of the body and carbon dioxide from the body cells to the lungs for exhalation. It carries nutrients from the gastrointestinal tract to body cells and hormones from endocrine glands to other body cells. Blood also transports heat and waste products to various organs for elimination from the body. 2. Regulation. Circulating blood helps maintain homeostasis of all body fluids. Blood helps regulate pH through the use of buffers (chemicals that convert strong acids or bases into weak ones). 3. Protection. Blood can clot (become gel-like), which protects against its excessive loss from the cardiovascular system after an injury. In addition, its white blood cells protect against disease by carrying on phagocytosis. Several types of blood proteins, including antibodies, interferons, and complement, help protect against disease in a variety of ways. Physical Characteristics of Blood The temperature of blood is 38°C about 1°C higher than oral or rectal body temperature, and it has a slightly alkaline pH ranging from 7.35 to 7.45. The color of blood varies with its oxygen content. When saturated with oxygen, it is bright red. When unsaturated with oxygen, it is dark red. Blood constitutes about 20% of extracellular fluid, amounting to 8% of the total body mass. In human the blood volume is 5 to 6 liters in an average-sized adult male and 4 to 5 liters in an averagesized adult female. Components of Blood Whole blood has two components: (1) blood plasma, and (2) formed elements, which are cells and cell fragments. Blood is about 45% formed elements and 55% blood plasma. Normally, more than 99% of the formed elements are red blood cells (RBCs). Pale, colorless white blood cells (WBCs) and platelets occupy less than 1% of the formed elements. Blood Plasma When the formed elements are removed from blood, a straw-colored liquid called blood plasma (or simply plasma) is left. Blood plasma is about 91.5% water and 8.5% solutes, most of which (7% by weight) are proteins. called plasma proteins. Hepatocytes (liver cells) synthesize most of the plasma proteins, which include the albumins (54% of plasma proteins), globulins (38%), and fibrinogen (7%). Certain blood cells develop into cells that produce gamma globulins, an important type of globulin. These plasma proteins are also called antibodies or immunoglobulins because they are produced during certain immune responses. Foreign substances (antigens) such as bacteria and viruses stimulate production of millions of different antibodies. An antibody binds specifically to the antigen that stimulated its production and thus disables the invading antigen. Besides proteins, other solutes in plasma include electrolytes, nutrients, regulatory substances such as enzymes and hormones, gases, and waste products such as urea, uric acid, creatinine, ammonia, and bilirubin. Formed Elements The formed elements of the blood include three principal components: red blood cells (RBCs), white blood cells (WBCs), and platelets. RBCs and WBCs are whole cells; platelets are cell fragments. Several distinct types of WBCs— neutrophils, lymphocytes, monocytes, eosinophils, and basophils—each with a unique microscopic appearance, carry out these functions. Following is the classification of the formed elements in blood: I. Red blood cells II. White blood cells A. Granular leukocytes (contain conspicuous granules that are visible under a light microscope after staining) 1. Neutrophils 2. Eosinophils 3. Basophils B. Agranular leukocytes (no granules are visible under a light microscope after staining) 1. T and B lymphocytes and natural killer (NK) cells 2. Monocytes III. Platelets The percentage of total blood volume occupied by RBCs is called the hematocrit, a hematocrit of 40 indicates that 40% of the volume of blood is composed of RBCs. The normal range of hematocrit for adult females is 38–46% (average 42%); for adult males, it is 40–54% (average 47%). FORMATION OF BLOOD CELLS called hemopoiesis or hematopoiesis. Red bone marrow becomes the primary site of hemopoiesis in the last 3 months before birth, and continues as the source of blood cells after birth and throughout life. RED BLOOD CELLS Red blood cells (RBCs) or erythrocytes contain the oxygen-carrying protein hemoglobin, which is a pigment that gives whole blood its red color. In human a healthy adult male has about 5.4 million red blood cells per microliter (µL) of blood, and a healthy adult female has about 4.8 million. RBCs are biconcave discs with a diameter of 7–8µm. RBC Physiology Each RBC contains about 280 million hemoglobin molecules. A hemoglobin molecule consists of a protein called goblin, composed of four polypeptide chains (two alpha and two beta chains); a ring like nonprotein pigment called a heme is bound to each of the four chains. At the center of each heme ring is an iron ion (Fe2+) that can combine reversibly with one oxygen molecule, allowing each hemoglobin molecule to bind four oxygen molecules. Each oxygen molecule picked up from the lungs is bound to an iron ion. As blood flows through tissue capillaries, the iron–oxygen reaction reverses. Hemoglobin releases oxygen, which diffuses first into the interstitial fluid and then into cells. Hemoglobin also transports about 23% of the total carbon dioxide, a waste product of metabolism. (The remaining carbon dioxide is dissolved in plasma or carried as bicarbonate ions.) Blood flowing through tissue capillaries picks up carbon dioxide, some of which combines with amino acids in the globin part of hemoglobin. As blood flows through the lungs, the carbon dioxide is released from hemoglobin and then exhaled. WHITE BLOOD CELLS Functions of WBCs In a healthy body, some WBCs, especially lymphocytes, can live for several months or years, but most live only a few days. During a period of infection, phagocytic WBCs may live only a few hours. WBCs are far less numerous than red blood cells; at about 5000–10,000 cells per microliter of blood, they are outnumbered by RBCs by about 700:1. Leukocytosis, an increase in the number of WBCs above 10,000/µL, is a normal, protective response to stresses such as invading microbes, strenuous exercise, anesthesia, and surgery. An abnormally low level of white blood cells (below 5000/µL) is termed leukopenia. Once pathogens enter the body, the general function of white blood cells is to combat them by phagocytosis or immune responses. To accomplish these tasks, many WBCs leave the bloodstream and collect at sites of pathogen invasion or inflammation. Once granular leukocytes and monocytes leave the bloodstream to fight injury or infection, they never return to it. Lymphocytes, on the other hand, continually recirculate — from blood to interstitial spaces of tissues to lymphatic fluid and back to blood. Only 2% of the total lymphocyte population is circulating in the blood at any given time; the rest is in lymphatic fluid and organs such as the skin, lungs, lymph nodes, and spleen. WBCs leave the bloodstream by a process termed emigration also called diapedesis, in which they roll along the endothelium, stick to it, and then squeeze between endothelial cells. Neutrophils and macrophages are active in phagocytosis, they can ingest bacteria and dispose of dead matter. Several different chemicals released by microbes and inflamed tissues attract phagocytes, a phenomenon called chemotaxis. Eosinophils leave the capillaries and enter tissue fluid. They are believed to release enzymes, such as histaminase, that combat the effects of histamine and other substances involved in inflammation during allergic reactions. Eosinophils also phagocytize antigen–antibody complexes and are effective against certain parasitic worms. A high eosinophil count often indicates an allergic condition or a parasitic infection. At sites of inflammation, basophils leave capillaries, enter tissues, and release granules that contain heparin, histamine, and serotonin. These substances intensify the inflammatory reaction and are involved in hypersensitivity (allergic) reactions Basophils are similar in function to mast cells, connective tissue cells that originate from pluripotent stem cells in red bone marrow. Like basophils, mast cells release substances involved in inflammation, including heparin, histamine, and proteases. Mast cells are widely dispersed in the body, particularly in connective tissues of the skin and mucous membranes of the respiratory and gastrointestinal tracts. Lymphocytes are the major soldiers in immune system battles. Most lymphocytes continually move among lymphoid tissues, lymph, and blood, spending only a few hours at a time in blood. Three main types of lymphocytes are B cells, T cells, and natural killer (NK) cells. B cells are particularly effective in destroying bacteria and inactivating their toxins. T cells attack viruses, fungi, transplanted cells, cancer cells, and some bacteria, and are responsible for transfusion reactions, allergies, and the rejection of transplanted organs. Immune responses carried out by both B cells and T cells help combat infection and provide protection against some diseases. Natural killer cells attack a wide variety of infectious microbes and certain spontaneously arising tumor cells. Monocytes take longer to reach a site of infection than neutrophils, but they arrive in larger numbers and destroy more microbes. On their arrival, monocytes enlarge and differentiate into wandering macrophages, which clean up cellular debris and microbes by phagocytosis after an infection. As you have already learned, an increase in the number of circulating WBCs usually indicates inflammation or infection. PLATELETS Between 150,000 and 400,000 platelets are present in each microliter of blood. Each is irregularly disc-shaped, 2–4µm in diameter, and has many vesicles but no nucleus. Their granules contain chemicals that, once released, promote blood clotting. Platelets help stop blood loss from damaged blood vessels by forming a platelet plug. Platelets have a short life span, normally just 5 to 9 days. Aged and dead platelets are removed by fixed macrophages in the spleen and liver. HEMOSTASIS Hemostasis, is a sequence of responses that stops bleeding. Three mechanisms reduce blood loss: (1) vascular spasm, (2) platelet plug formation, and (3) blood clotting (coagulation). Vascular Spasm When arteries or arterioles are damaged, the circularly arranged smooth muscle in their walls contracts immediately, a reaction called vascular spasm. This reduces blood loss for several minutes to several hours, during which time the other hemostatic mechanisms go into operation. Platelet Plug Formation Considering their small size, platelets store an impressive array of chemicals. Within many vesicles are clotting factors, ADP, ATP, Ca2+, and serotonin. Also present are enzymes that produce thromboxane A2, a prostaglandin; fibrin-stabilizing factor, which helps to strengthen a blood clot; lysosomes; some mitochondria; membrane systems that take up and store calcium and provide channels for release of the contents of granules; and glycogen. Platelet plug formation occurs as follows: ●1 Initially, platelets contact and stick to parts of a damaged blood vessel, such as collagen fibers of the connective tissue underlying the damaged endothelial cells. This process is called platelet adhesion. ●2 Due to adhesion, the platelets become activated, and extend many projections that enable them to contact and interact with one another. .●3 They release ADP so become sticky, and the stickiness recruits and activates other platelets to adhere to the originally activated platelets. This gathering of platelets is called platelet aggregation. Eventually, the accumulation and attachment of large numbers of platelets form a mass called a platelet plug. Blood Clotting The process clotting or coagulation, is a series of chemical reactions that culminates in formation of fibrin threads. Thrombosis—clotting in an undamaged blood vessel. Clotting involves several substances known as clotting (coagulation) factors. These factors include calcium ions (Ca2+), several inactive enzymes that are synthesized by hepatocytes (liver cells) and released into the bloodstream, and various molecules associated with platelets or released by damaged tissues. Clotting can be divided into three stages; ●1 Two pathways, called the extrinsic pathway and the intrinsic pathway, lead to the formation of prothrombinase. Once prothrombinase is formed, the steps involved in the next two stages of clotting are the same for both the extrinsic and intrinsic pathways, and together these two stages are referred to as the common pathway. ●2 Prothrombinase converts prothrombin (a plasma protein formed by the liver) into the enzyme thrombin. ●3 Thrombin converts soluble fibrinogen (another plasma protein formed by the liver) into insoluble fibrin. Fibrin forms the threads of the clot. s The Extrinsic Pathway The extrinsic pathway of blood clotting is so named because a tissue protein called tissue factor (TF), also known as thromboplastin, leaks into the blood from cells outside (extrinsic to) blood vessels and initiates the formation of prothrombinase. In the presence of Ca2+, TF begins a sequence of reactions that ultimately activates clotting factor X. Once factor X is activated, it combines with factor V in the presence of Ca2+ to form the active enzyme prothrombinase, completing the extrinsic pathway. The Intrinsic Pathway The intrinsic pathway of blood clotting is so named because its activators are either in direct contact with blood or contained within (intrinsic to) the blood; outside tissue damage is not needed. If endothelial cells become roughened or damaged, blood can come in contact with collagen fibers in the connective tissue around the endothelium of the blood vessel. In addition, trauma to endothelial cells causes damage to platelets, resulting in the release of phospholipids by the platelets. Contact with collagen fibers (or with the glass sides of a blood collection tube) activates clotting factor XII which begins a sequence of reactions that eventually activates clotting factor X. Platelet phospholipids and Ca2+ can also participate in the activation of factor X. Once factor X is activated, it combines with factor V to form the active enzyme prothrombinase (just as occurs in the extrinsic pathway), completing the intrinsic pathway. The Common Pathway The formation of prothrombinase marks the beginning of the common pathway. In the second stage of blood clotting, prothrombinase and Ca2_ catalyze the conversion of prothrombin to thrombin. In the third stage, thrombin, in the presence of Ca2_, converts fibrinogen, which is soluble, to loose fibrin threads, which are insoluble. Thrombin also activates factor XIII (fibrin stabilizing factor), which strengthens and stabilizes the fibrin threads into a sturdy clot. Plasma contains some factor XIII, which is also released by platelets trapped in the clot. Clot Retraction Once a clot is formed, it plugs the ruptured area of the blood vessel and thus stops blood loss. Clot retraction is the consolidation or tightening of the fibrin clot. The fibrin threads attached to the damaged surfaces of the blood vessel gradually contract as platelets pull on them. BLOOD GROUPS AND BLOOD TYPES The surfaces of erythrocytes contain a genetically determined assortment of antigens composed of glycoproteins and glycolipids. These antigens, called agglutinogens occur in characteristic combinations. Based on the presence or absence of various antigens, blood is categorized into different blood groups. ABO Blood Group The ABO blood group is based on two glycolipid antigens called A and B. People whose RBCs display only antigen A have type A blood. Those who have only antigen B are type B. Individuals who have both A and B antigens are type AB; those who have neither antigen A nor B are type O. Blood plasma usually contains antibodies called agglutinins that react with the A or B antigens if the two are mixed. These are the anti-A antibody, which reacts with antigen A, and the anti-B antibody, which reacts with antigen B. You do not have antibodies that react with the antigens of your own RBCs, but you do have antibodies for any antigens that your RBCs lack. For example, if your blood type is B, you have B antigens on your red blood cells, and you have anti-A antibodies in your blood plasma. Transfusions A transfusion is the transfer of whole blood or blood components (red blood cells only or blood plasma only) into the bloodstream or directly into the red bone marrow. However, the normal components of one person’s RBC plasma membrane can trigger damaging antigen–antibody responses in a transfusion recipient. In an incompatible blood transfusion, antibodies in the recipient’s plasma bind to the antigens on the donated RBCs, which causes agglutination, or clumping, of the RBCs. Agglutination is an antigen–antibody response in which RBCs become cross-linked to one another. When these antigen–antibody complexes form, they activate plasma proteins of the complement family. In essence, complement molecules make the plasma membrane of the donated RBCs leaky, causing hemolysis or rupture of the RBCs and the release of hemoglobin into the blood plasma. The liberated hemoglobin may cause kidney damage by clogging the filtration membranes. Consider what happens if a person with type A blood receives a transfusion of type B blood. The recipient’s blood (type A) contains A antigens on the red blood cells and anti-B antibodies in the plasma. The donor’s blood (type B) contains B antigens and anti-A antibodies. In this situation, two things can happen. First, the anti- B antibodies in the recipient’s plasma can bind to the B antigens on the donor’s erythrocytes, causing agglutination and hemolysis of the red blood cells. Second, the anti-A antibodies in the donor’s plasma can bind to the A antigens on the recipient’s red blood cells, a less serious reaction because the donor’s anti-A antibodies become so diluted in the recipient’s plasma that they do not cause significant agglutination and hemolysis of the recipient’s RBCs. People with type AB blood do not have anti-A or anti-B antibodies in their blood plasma. They are sometimes called universal recipients because theoretically they can receive blood from donors of all four blood types. They have no antibodies to attack antigens on donated RBCs. People with type O blood have neither A nor B antigens on their RBCs and are sometimes called universal donors because theoretically they can donate blood to all four ABO blood types. Type O persons requiring blood may receive only type O blood. In practice, use of the terms universal recipient and universal donor is misleading and dangerous. Blood contains antigens and antibodies other than those associated with the ABO system that can cause transfusion problems. Thus, blood should be carefully crossmatched or screened before transfusion. Rh Blood Group The Rh blood group is so named because the Rh antigen, called Rh factor, was first found in the blood of the Rhesus monkey. People whose RBCs have Rh antigens are designated Rh+ (Rh positive); those who lack Rh antigens are designated Rh- (Rh negative). Normally, blood plasma does not contain anti-Rh antibodies. If an Rh- person receives an Rh+ blood transfusion, however, the immune system starts to make anti-Rh antibodies that will remain in the blood. If a second transfusion of Rh+ blood is given later, the previously formed anti-Rh antibodies will cause agglutination and hemolysis of the RBCs in the donated blood, and a severe reaction may occur. Plasma Proteins Plasma proteins are the major components of plasma. They consist of albumin, globulin, and fibrinogen. Most plasma proteins are manufactured in the liver. Some plasma proteins (immunoglobulins/ antibodies) are made by specific lymphocytes. The plasma proteins have varied functions. They serve to maintain the pH of the blood at 7.4. Some protein components are antibodies that recognize specific antigens. A few of the clotting factors are proteins. Some proteins serve as transport carriers for hormones, metals, amino acids, fatty acids, enzymes, and drugs. Because the protein molecules are large and the capillary walls are impermeable to them, substances escape filtration by the kidneys and stay longer in the blood when they are bound to proteins. The protein fractions exert an osmotic force of about 25 mm Hg across the capillary wall. This force tends to pull water into the blood from the surrounding fluid compartments, such as the interstitial compartment, and maintains the blood volume. In individuals with protein deficiency, the reduction of this force is responsible for the edema that develops. Cerebrospinal fluid Embedded within the brain are four ventricles or chambers that form a continuous fluid-filled system. In the roof of each of these ventricles is a network of capillaries referred to as the choroid plexus. It is from the choroid plexuses of the two lateral ventricles (one in each cerebral hemisphere) that cerebrospinal fluid (CSF) is primarily derived. Due to the presence of the blood–brain barrier, the selective transport processes of the choroid plexus determine the composition of the CSF. Therefore, the composition of the CSF is markedly different from the composition of the plasma. However, the CSF is in equilibrium with the interstitial fluid of the brain and contributes to the maintenance of a consistent chemical environment for neurons, which serves to optimize their function. The CSF flows through the ventricles, downward through the central canal of the spinal cord, and then upward toward the brain through the subarachnoid space that completely surrounds the brain and spinal cord. As the CSF flows over the superior surface of the brain, it leaves the subarachnoid space and is absorbed into the venous system. Although CSF is actively secreted at a rate of 500 ml/day, the volume of this fluid in the system is approximately 140 ml. Therefore, the entire volume of CSF is turned over three to four times per day. The one-way flow of the CSF and the constant turnover facilitate its important function of removing potentially harmful brain metabolites. The CSF also protects the brain from impact by serving as a shockabsorbing system that lies between the brain and its bony capsule. Finally, because the brain and the CSF have about the same specific gravity, the brain floats in this fluid. This reduces the effective weight of the brain from 1400 g to less than 50g and prevents compression of neurons on the inferior surface of the brain. The Lymphatic System In general, the fluid moving out of the capillaries exceeds that entering it from the interstitial fluid compartment. Also, large protein particles tend to accumulate in this compartment. These proteins may be particles that have leaked from the blood into the capillaries, cell waste products, or remains of dead tissue. Being large, these proteins cannot be easily removed by the capillaries, however, another mechanism—the lymphatic system—is in place to remove excessive fluid and proteins. FUNCTIONS OF THE LYMPHATIC SYSTEM The function of the lymphatic system is to return excess fluid and protein from the interstitial fluid compartment back into the blood circulation. If the protein is not returned to the blood, the plasma colloid osmotic pressure will drop, and it will not be possible for fluid to stay inside the circulatory system. Defense is another important function of this system. Lymphoid tissue is responsible for the production, maintenance, and distribution of lymphocytes, a class of white blood cells that participates in defense. The white blood cells in the lymphatic system remove foreign agents that have entered the interstitial region. In the intestine, lymphatics help carry fat and large particles to the liver. In the kidney, adequate lymphatic flow is required for concentrating the urine. Lymph Vessels The lymphatic system is similar to the cardiovascular system because it, too, has vessels, often called lymphatics. Lymphatics are present in almost all the regions of the body; however, they are absent from the central nervous system and such regions as the cornea, lens, cartilage, and epithelium that lack a blood supply. The smallest vessels—the lymphatic capillaries— arise as blind-ended tubes in the interstitial spaces. These capillaries have thinner walls, and they are larger than blood capillaries. The lymph capillaries are highly permeable and allow large particles to easily enter the vessel. The endothelial cells lining the capillaries have gaps that allow the particles to enter. In addition, the cells overlap with each other, with the overlap acting as one-way valves. Anchoring filaments—proteins attached to the endothelial cells—also help adjust the width of the gaps. When there is more fluid inside the lymph capillaries, the width of the gap becomes smaller, allowing less fluid in and preventing backflow of fluid. When there is more fluid in the interstitial compartment, the anchoring filaments are pulled and the gap widens, allowing more fluid to enter the capillaries. The lymph capillaries in the intestines (lacteals) are located in the center of the villi. The lymph in the lacteal carries a high fat content, giving the lymph a creamy, white appearance. Lymph flowing through the lacteals is referred to as chyle. From the periphery, the networks of capillaries join and rejoin others to form larger lymphatic vessels. The lymphatic vessels resemble veins, with an endothelium, smooth wall muscles, and adventitia. The inner lining of these large vessels is thrown into folds to form valves. Lymph vessels have numerous valves located every few millimeters, giving the vessels a beaded appearance. At various intervals, the lymph vessels open into lymphatic tissue called lymph nodes. Lymphatic vessels from the lymph nodes join others and progressively become larger until they communicate with two collecting vessels, the thoracic duct or left lymphatic duct, the largest of these vessels, and the right lymphatic duct. The lower end of the thoracic duct is enlarged and is known as the cisterna chyli. The thoracic duct and the right lymphatic duct are located in the thoracic cavity. The thoracic duct is about 38–45 cm (15–17.7 in) long and runs parallel to the vertebral column. The right lymphatic duct is much shorter, about 1.5 cm (0.6 in) long. Both ducts open into the blood vessels in the neck (on the left and right side, respectively) at the junction of the subclavian and internal jugular vein. Thus, lymph is emptied into the blood circulation. The thoracic duct collects lymph from the left side of the body and from the right side of the body inferior to the diaphragm. The right lymphatic duct collects The thoracic duct and the right lymphatic duct are located in the thoracic cavity. The thoracic duct is about 38– 45 cm (15–17.7 in) long and runs parallel to the vertebral column. The right lymphatic duct is much shorter, about 1.5 cm (0.6 in) long. Both ducts open into the blood vessels in the neck (on the left and right side, respectively) at the junction of the subclavian and internal jugular vein. Thus, lymph is emptied into the blood circulation. The thoracic duct collects lymph from the left side of the body and from the right side of the body inferior to the diaphragm. The right lymphatic duct collects Lymph and Lymph Flow The lymph or lymph fluid, a clear, pale yellow fluid, is the overflow fluid from the tissue spaces with the same composition as the interstitial fluid. It carries large proteins and waste from different parts of the body. Lymph vessels, unlike blood vessels, do not have extensive smooth muscle around them to aid the flow. Also, the lymphatic system does not have a pump equivalent to the heart to circulate the fluid. This system, therefore, must rely on other mechanisms to move the fluid toward the neck. Lymph vessels have one-way valves to help direct the fluid. Lymph is also propelled to a large extent by the passive and active movements of skeletal muscles. In addition, the pulsation of arteries lying close to the lymph vessels helps propel the lymph. Another important mechanism that draws the lymph upward is the respiratory movements. When a person inspires, the pressure drops in the thorax and increases in the abdomen. This difference in pressure is sufficient to “suck” the lymph into the thorax and the venous system. Changes in posture, passive compression, and massage can also aid lymph flow. The smooth wall muscles of the lymphatic vessels also help move lymph by contracting when distended. The rate of flow increases with physical activity. It has been estimated that about 2–4 liters of lymphatic fluid and the equivalent of 25% to 50% of the total circulating plasma protein is returned to the circulation every day.