Lab for types of reactions
advertisement

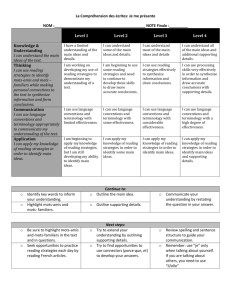
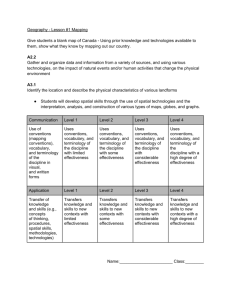
Lab for types of reactions 1. What curriculum expectations does this lab meet? Overall Expectations C2. Investigate, through inquiry, the characteristics of chemical reactions; C3. Demonstrate an understanding of the general principles of chemical reactions, and various ways to represent them. Specific Expectations C2.2 Construct molecular models to illustrate the structure of molecules in simple chemical reactions (e.g., C + O2 ➞ CO2; 2H2 + O2 ➞ 2H2O), and produce diagrams of these models. [PR, C] C2.3 Investigate simple chemical reactions, including synthesis, decomposition, and displacement reactions, and represent them using a variety of formats (e.g., molecular models, word equations, balanced chemical equations). [PR, AI, C] C3.4 Write word equations and balanced chemical equations for simple chemical reactions. (e.g., 2H2 + O2 ➞ 2H2O) C3.5 Describe, on the basis of observation, the reactants in and products of a variety of chemical reactions, including synthesis, decomposition, and displacement reactions. (e.g., reactions occurring when magnesium burns or in the production of oxygen from hydrogen peroxide; the reaction of iron and copper sulphate; reactions occurring when fossil fuels burn) 2. What safety instructions would you give students before/during and after the lab? Safety Instructions 1. Conduct yourself in a responsible manner at all times in the laboratory. 2. Follow all written and verbal instructions carefully. If you do not understand a direction or part of a procedure, ASK YOUR TEACHER BEFORE PROCEEDING WITH THE ACTIVITY. 1. Do not eat food, drink beverages, or chew gum in the laboratory. Do not use laboratory glassware as containers for food or beverages. 2. Be prepared for your work in the laboratory. Read all procedures thoroughly before entering the laboratory. Never fool around in the laboratory. Horseplay, practical jokes, and pranks are dangerous and prohibited. 3. Always work in a well-ventilated area. 4. Be alert and proceed with caution at all times in the laboratory. Notify the teacher immediately of any unsafe conditions you observe. 5. Experiments must be personally monitored at all times. Do not wander around the room, distract other students, startle other students or interfere with the laboratory experiments of others. 6. Know the locations and operating procedures of all safety equipment including: first aid kit(s), and fire extinguisher. Know where the fire alarm and the exits are located. 7. Know what to do if there is a fire drill during a laboratory period; containers must be closed, and any electrical equipment turned off. 8. Any time chemicals, heat, or glassware are used, students will wear safety goggles. NO EXCEPTIONS TO THIS RULE! 9. Report any accident (spill, breakage, etc.) or injury (cut, burn, etc.) to the teacher immediately, no matter how trivial it seems. Do not panic. 10. If you or your lab partner is hurt, immediately (and loudly) yell out the teacher's name to get the teacher's attention. Do not panic. 11. Never handle broken glass with your bare hands. Use a brush and dustpan to clean up broken glass. Place broken glass in the designated glass disposal container. 12. Never force glass tubing into rubber stoppers. 13. Students should properly note odors or fumes with a wafting motion of the hand. 14. Confine long hair and loose clothing. Laboratory aprons should be worn. 15. Students should avoid transferring chemicals they have handled to their faces. 16. Examine glassware before each use. Never use chipped, cracked, or dirty glassware. 17. If you do not understand how to use a piece of equipment, ASK THE TEACHER FOR HELP! 18. Do not place hot apparatus directly on the laboratory desk. Always use an insulated pad. Allow plenty of time for hot apparatus to cool before touching it. 19. Follow the give instructions to disposal of the chemicals appropriately. 3. What prior knowledge do you need to be able to lead the lab? They will need to know the different types of reactions (synthesis, decomposition, combustion, single displacement of cation or anion, or double displacement and neutralization reaction) How to write and balanced chemical equation for reaction They will know how to write the chemical names & formula for the products of a reaction 4. What is the purpose of the lab? Is it to introduce a concept? Reinforce? Lab skills? etc. The purpose of this lab is to reinforce the concept of the types of chemical reactions and to reinforce this through a lab activity. 5. How will you assess this lab? (Please make specific reference to KICA) Category Use of critical/creative thinking processes, skills, and strategies (e.g., analysing, interpreting, problem solving, evaluating, forming and justifying conclusions on the basis of evidence ) Level 1 uses critical/ creative thinking processes, skills, and strategies with limited effectiveness Level 2 uses critical/ creative thinking processes, skills, and strategies with some effectiveness Level 3 uses critical/ creative thinking processes, skills, and strategies with considerable effectiveness Level 4 uses critical/ creative thinking processes, skills, and strategies with a high degree of effectiveness Application of knowledge and skills (e.g., concepts and processes, safe use of equipment, scientific investigation skills) in familiar contexts Use of conventions, vocabulary, and terminology of the discipline in oral, visual, and/or written forms (e.g., symbols, formulae, scientific notation, SI units) Applies knowledge and skills in familiar contexts with limited effectiveness Applies knowledge and skills in familiar contexts with some effectiveness Applies knowledge and skills in familiar contexts with considerable effectiveness Applies knowledge and skills in familiar contexts with a high degree of effectiveness uses conventions, vocabulary, and terminology of the discipline with limited effectiveness uses conventions, vocabulary, and terminology of the discipline with some effectiveness uses conventions, vocabulary, and terminology of the discipline with considerable effectiveness uses conventions, vocabulary, and terminology of the discipline with a high degree of effectiveness 6. How could you modify this lab for enriched students? students with IEPs? ELL? etc. Make basic accommodations for special needs students by providing these students with extra time, computer options, strategic seating, assistive technology, etc., as outlined in their Individual Education Plans (IEPs). For the ELL student will give them a vocabulary sheet with the explanation of difficult word or concepts, and the use of pictures in lab safety sheet. 7. What will the students gain from participating in this activity? The students will gain a better understanding of the general principles of chemical reactions, and various ways to represent them. They will also gain more experience with writing word equations and balanced chemical equations for simple chemical reactions.