jec12219-sup-0001-AppendixS1-FigureS1
advertisement

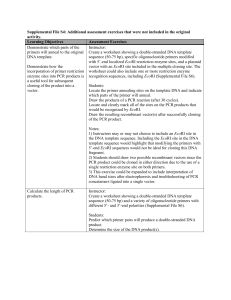
No signs of meristem senescence in old Scots pine Maurizio Mencuccini, Marta Oñate, Josep Peñuelas, Laura Rico, Sergi Munné-Bosch Supplementary Materials Hormonal analyses Concentrations of the cytokinins, zeatin, zeatinriboside, isopentenyladenosine, isopenteniladenine and dihydrozeatinriboside, the auxin, indole-3-acetic acid, the ethylene precursor, 1-amino-cyclopropane-1-carboxylic acid, together with abscisic acid, jasmonic acid, salicylic acid and gibberellins 4 and 24were determined by UPLC-MS/MS (ultra-high performance liquid chromatography coupled to tandem mass spectrometry) following the method described by Müller and Munné-Bosch (2011). In brief, leaf samples were ground in liquid nitrogen and, after addition of a mixed deuterated pool of all compounds as an internal standard, they were extracted with methanol: isopropanol: glacial acetic acid (40:59:1) using sonication. The fresh extracts were filtered through a 0.22 mm PTFE filter (Waters), and immediately injected into the UPLC-MS/MS system. The UPLC system consisted of an Acquity Waters binary pump equipped with an autosampler and a UV detector. For analysis of the extracts, a HALO C18 column was used with a binary solvent system comprising acetonitrile and deionized water, both containing 0.005% (v/v) glacial acetic acid. Separations were performed using a gradient of increasing acetonitrile content, at a constant flow rate of 0.4 ml min-1. MS/MS analyses were performed on an API 3000 triple quadrupole mass spectrometer (PE Sciex) using the Turbo Ion spray source in negative ion mode. MRM acquisition and quantification by MS/MS was performed as described (Müller and Munné-Bosch, 2011). Results were corrected taking into account the specific recovery rates using the internal deuterated standard (Müller and Munné-Bosch, 2011). HPLC Analyses Leaf samples were ground in liquid nitrogen and extracted with ice-cold methanol using a Branson 2510 ultrasonic cleaner for 45 min. The samples were then centrifuged for 15 min at 4ºC and transferred to vials for analysis. The HPLC analysis was carried out as described in Amaral et al.(2005). In brief, the HPLC equipment consisted of an integrated system with a Waters 600 controller pump, a Waters 714 plus auto-samplerand an FP-1520 fluorescence detector. Tocopherols were separated on an Inertsil 100A normal-phase column operating at room temperature. Quantification was based on the fluorescence signal response compared with authentic standards of each compound (Sigma-Aldrich). Methylation-sensitive amplification polymorphism (MSAP) analysis Approximately 20 mg leaf tissue were ground in liquid nitrogen using a mixed mill (Tissue Lyser, Qiageninc, Valencia, CA, USA) and two glass beads or one tungsten carbide bead, depending on the samples hardness. Disruption was performed in two 1-2 minute high-speed (20-30 Hz) shaking steps. Genomic DNA was extracted from ground tissue using a DNeasy Plant Mini Kit (Qiagen) and quantified using a NanoDrop ND-1000 spectrophotometer running software v3.0.1 (NanoDrop Technologies, Wilmington, DE, USA) following the manufacturer’s instructions. To improve the MSAP detection method previously described (Reyna-López et al., 1997), we used a fluorescent labelling system which is more sensitive, faster and safer than other detection methods like radioisotopes or silver stain. One µg of genomic DNA was subjected to double digestion using 10 units of EcoRI (NEB, Beverly, MA, USA) and 10 units of one of the isoschizomer enzymes (MspI or HpaII) (NEB, Beverly, MA, USA) in 50 l of the manufacturers buffer (composition confidential) and incubated overnight at 37 °C. The ligation reaction contained 20 l digested solution, 5 pmolEcoRI adapter, 50 pmolMspI/HpaII adapter, 1 unit of T4 DNA ligase (Roche Diagnostics, Barcelona, Spain) and 3 µl of the manufacturers buffer (composition confidential). Adaptors were ligated 3h at 22ºC and overnight at 4ºC. The core sequence of the EcoRI adapters and primers are those used in the original AFLP protocol by Vos et al. (1995) and the core sequence of the MspI/HpaII adapters and primers are those used in Xu et al. (2000). All adapters and primers were synthesized by METABION (Martinsried, Germany). Two µl of the digestion/ligation mix was used as the template for the pre-amplification. PCR was performed with 30 ngEco-N and 30 ng of MspI/HpaII-N primers with 1 unit Taq DNA Polimerase (Roche Diagnostics)and 0.4mM each dATP, dCTP, dGTP, dTTP with 2.5 mMMgCl2 in a total of 20 µl of the manufacturers buffer (composition confidential). The PCR programme consisted of 29 cycles of (94 °C for 30 s, 56 °C for 60 s, 72 °C for 60 s). PCR products were diluted 1:20 with sterile H2O. Twenty Eco-ANN/MspI/HpaII-CNN primer combinations were tested for selective amplification (where N represents any nucleotide). Eco-NNN primer extensions were: AAG, AGG, ACG and ACC. MspI/HpaII-NNN primer extensions were: TTC, TAG, CAG, CAC, CTG, CTC, CTA and CAT. The combinations finally used were Eco-AGC + MspI/HpaIICAC and Eco-AGC+ MspI/HpaII-CTC, based on clear banding pattern, polymorphism and reproducibility between replicates. The Eco-NNN primer carried a VIC fluorochrome (Applied Biosystems – ABI, Foster City, CA, USA). Five µl of the diluted PCR products was used as the template for the selective amplification. PCR was performed using 10 ngEcoANN primer and 30 ngMspI/HpaII-CNN primer with 0.5 units Taq DNA Polimerase (Roche Diagnostics)and 0.2 mM each of dATP, dCTP, dGTP, dTTP and 2 mM MgCl2 in a total of 10 µl of the manufacturers buffer. The PCR programme consisted of 37 cycles in total: 14 cycles of (94 °C for 30 s, t °C for 30 s, 72 °C for 60 s, where t drops from 65 to 56 in 0.7°C steps), followed by 23 cycles of (94 °C for 30 s, 56 °C for 30 s, 72 °C for 60 s). For the analysis of the selective MSAP-PCR products, 2 l of PCR product were mixed with 12 l Hi-Di Formamide (ABI) and 0.4 l Liz-600 size standard (ABI). Samples were denatured at 94ºC for 3 min and electrophoresis performed using an ABI3130xl genetic analyser according to the manufacturer’s instructions. Fragment sizes were determined with reference to the size standard using GeneMarker v 1.85 (Softgenetics LLC). Fragment presence or absence was scored by hand by one person and subsequently checked by a second investigator without reference to sample ID. A binary matrix of band presence/absence was then created. Each set of 96 reactions included two positive (known genotype duplicates) and two negative (H2O or PCR mix without DNA) controls carried from restriction digest through to selective MSAP-PCR. Any band occurring in a sample was excluded from the analysis if it also occurred in a negative control. Individuals that repeatedly failed to amplify or show poor amplification were eliminated from the dataset. For a given DNA sample, the presence of hyper-methylated loci could only be detected from the loss of a fragment in both MSAP-profiles when comparing with other individuals genotypes in a polymorphic loci; however HpaII and MspI cannot distinguish full methylation state of both cytosines on a monomorphic locus either than other states of the CCGG sites as hemi-methylation of the internal cytosine (CmCGG/GGCC), thus leading to an underestimate of the total genome methylation level(Salmon et al., 2008). A total of 298 MSAP loci were detected resulting in a total of 14988 fragments resolved in DNA extracted from 53 individual samples (young and mature leaves from 27 ortets over two dates, one missing sample). Regularized Generalised Canonical Correlation Analysis Before using RGCCA, all our data were scaled to zero mean and unit variance. We employed both the centroid and the factorial scheme as optimization criteria (with very similar results), and we used the new mode A for the calculation of the shrinkage coefficients of the sample covariance matrices, which is the most appropriate when block components represent underlying latent variables (Fornell and Bookstein 1982). The confidence intervals of the correlation coefficients among block components (the latent variables) and between block variables (the actual measurements for each group) and the corresponding block component were determined by random permutation of the values of each variable in the datasets 1,000 times with replacement and by extracting the relevant 0.025 and the 0.975 quantiles from the correspondent distribution. Model goodness of fit was calculated using the concept of AVE, the average explained variance at the various hierarchical levels, i.e., values of AVE were obtained for each relationship between a block component and internal block variables, as an overall weighted mean of all the relationships among block components and internal block variables (outer model AVE) and for the combined relationships among all block components (inner model AVE). An overall estimate of global model fit was obtained using the geometric means of the AVEs of the inner and of the outer models (Tenenhaus and Tenenhaus 2011). Table S1. Description of theMethylation-Sensitive Amplification Polymorphism analysis (MSAP) to detect the methylation state of genomic DNA by differential cleavage of the restriction site (CCGG) and by the restriction isoschizomer pair HpaII and MspI RESTRICTION AMPLIFIED MSAP ENZYME PATTERN ACTIVITY HpaII MspI EcoRI/HpaII EcoRI/MspI METHYLATION STATUS CCGG SITE Non-methylated loci CCGG / CCGG + + 1 1 CmCGG / GGCmC - + 0 1 + - 1 0 Fully-methylated loci (both strands methylated) Hemi-methylated loci (only one strand methylated) mCCGG / GGCC mCmCGG / 0 0 GGCmCm † Notes: (mC) cytosine methylated; (-) inactive, sensitive to methylation status; (+) active; (1) band present; (0) band absent Hyper-methylated loci Fig. S1. Relationship between meristematic age (X axis) and various other growth and reproductive parameters of the grafted trees.A) height; B) diameter; C) height growth rate during the last four years; D) number of cones per tree; E) number of seeds per cone and F) arcsine-transformed percentage germination of full seeds. Symbols ‘NS’ and ‘0.05’ in the panels indicate the significance of the regression slopes at, respectively, P>0.05 and P<0.05.