130 0 C down 130 0 C up 150 0 C down 150 0 C up 200 0 C down
advertisement

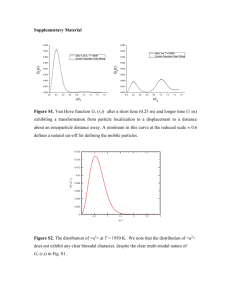
Supporting Information Simultaneously synthesis nano and micro Ag particles and their application as a die-attachment material Jinting Jiua,*, Hao Zhanga,b, Shunsuke Kogaa,b, Shijo Nagaoa, Yasuha Izumi a, Katsuaki Suganumaa a Institute of Scientific and Industrial Research, Osaka University, Ibaraki, Osaka 567-0047, Japan b Graduate School of Engineering, Osaka University, Ibaraki, Osaka 567-0047, Japan Email: jiu@eco.sanken.osaka-u.ac.jp Abstract A simple and large-scale two-steps polyol process was used to simultaneously synthesize nano and micro Ag particle pastes, which were used to joint Ag-plated Cu sheets. The size and size distribution of the Ag particles and less organics remainder determined the shear strength and conductivity of the Ag paste. A shear strength of over 40 MPa and a resistivity of 5.1 cm were achieved at 200°C using a paste with nano-Ag below 800 nm and micro-Ag of 1.5-4.2 m in diameter under a minor sintering pressure of 0.4 MPa. The Ag paste included too huge and much micro-Ag particles, for example from 2-5.2 m only gave maximum strengths of 30 MPa due to inhomogeneous sintering and the presence of large voids. These results are superior to those obtained with nanoparticle Ag pastes and suggested that suitable submicrometer Ag particles replacing nanoparticles Ag will be an excellent die-attachment material in microelectronic packaging. Fig. S1. The preparation schematic diagram of Ag pastes. In the first step, 0.5 g of poly(vinylpyrrolidone) (PVP, MW = 360000) and 1.0 g of silver nitrate (AgNO3) were dissolved to 50 g of ethylene glycol (EG) at room temperature (RT), and then reacted at 150°C for 1 h. During the first 30 min of the reaction, a different solution comprising 1.0 g AgNO3 and 50 g EG was dropped in. Acetone and ethanol were then used to wash the precipitate. Finally, the Ag nanoparticles were redispersed as seeds in ethanol (2.5 wt%) for future use. In the second step, 1.5 g of AgNO3 was dissolved in 50 g EG to obtain a clear solution which was slowly dropped into a solution, prepared and heated in advance, containing 5.0 g of seed solution and 50 g EG at 130, 150 and 200°C, respectively. The solution was then left to react for an additional 30 min. Finally, the precipitation was washed three times with acetone and ethanol to obtain pure Ag particle precipitates, which was respectively mixed with a small amount of EG to obtain Ag pastes. (a) (b) (c) (d) Fig. S2a. Ag nanoparticles prepared with one-step. The detail is 0.5 g of PVP was first added to 50 g of ethylene glycol (EG) and completely dissolved using magnetic stirring at room temperature. Afterwards, 1.0 g of silver nitrate (AgNO3) was added to the PVP solution and reacted at 150°C for 1 hour. During the reaction, another solution including 2.5 g AgNO3 and 50 g EG was dropped into the solution to increase the size. The dropping time was 1 hours, after that the solution is left to react another 1 hour. At last the precipitation was washed with acetone and ethanol for three times to get the pure Ag nanoparticles precipitation. The diameter of Ag nanoparticles is about 300-500 nm with irregular morphology (a). Comparing those Ag seeds (Fig. S1b), these Ag size was slightly increased with clear sharp edge in these nanoparticles due to long reaction time. However, these Ag particles are far smaller than those Ag particles prepared with two-steps process (Fig. S1c). Fig. S1d shows the Ag particles prepared without PVP dispersant at same conditions with one-step. Fig. S3. Traces of DTA and TG for Ag pastes prepared at different reaction temperature. These Ag pastes have been dried at 100 0C for two weeks and then were analyzed with TG/DTA. Almost no weight-loss was observed in these Ag pastes. It is noted that the DTA curves have been changed comparing those curves before the two-week dry process (Fig. 3b). A clear exothermic peak appeared in the figure 3b has been shifted to high temperature, which might implied that Ag particles have been aggregated into big particles due to long-time dry process. These big aggregation particles were sintered by surface diffusion and reallocation will need a high temperature leading to an exothermic peak. These results also suggested that the organic compounds might correspond to the low-temperature sintering of these Ag pastes. (a) (b) (c) Fig. S4. The SEM images of Ag particles prepared at 130 (a), 150 (b) and 200 0C after drying at 100 0C. 0 130 C down 0 150 C down 0 200 C down 0 130 C up 0 150 C up 0 200 C up Fig. S5. The photos of joints after shear test. For Ag past prepared at 130 0C, it is clear that the Ag paste has been left on the two sides, i.e. two copper sheets, which suggested that fracture occurred in the Ag pastes not near the interface of copper and pastes. The result clearly implied these Ag pastes have strong joint which has been confirmed with high shear strength. For other two pastes, the fracture surface was near interface between Ag paste and substrates, which implied that the strength was weak due to non-uniform sintering of Ag pastes.