Determining Hydrogen Ion Concentration of Strong & Weak Acids
advertisement

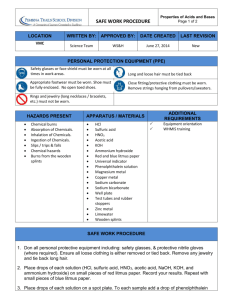
SAFE WORK PROCEDURE LOCATION VMC WRITTEN BY: APPROVED BY: Science Team Determining Hydrogen Ion Concentration of Strong and Weak Acids Page 1 of 2 DATE CREATED WS&H LAST REVISION June 27, 2014 New PERSONAL PROTECTION EQUIPMENT (PPE) Safety glasses or face shield must be worn at all times in work areas. Long and loose hair must be tied back Appropriate footwear must be worn. Shoe must be fully enclosed. No open toed shoes. Close fitting/protective clothing must be worn. Remove strings hanging from pullovers/sweaters. Rings and jewelry (long necklaces / bracelets, etc.) must not be worn. HAZARDS PRESENT Chemical burns Absorption of Chemicals. Inhalation of Chemicals. Ingestion of Chemicals. Slips / trips & falls Chemical hazards ADDITIONAL REQUIREMENTS APPARATUS / MATERIALS Test tubes and rack Graduated cylinder Pipet Marking pencil Distilled water 0.1M HCl 1.0M acetic acid Methyl orange Orange IV Equipment orientation WHMIS training SAFE WORK PROCEDURE 1. Don all personal protective equipment including: safety glasses, & protective nitrile gloves (where required). Ensure all loose clothing is either removed or tied back. Remove any jewelry and tie back long hair. 2. Obtain 5 ml of HCl in a small beaker. Pipette 2 ml of the HCl into a clean test tube. This is Concentration 1 Set 1. Pipette 2 ml of the HCl into another clean test tube. This is Concentration 1 Set 2. 3. Label the test tubes. Save the remaining 1 ml of HCl for concentration 2. 4. Using a 10 ml graduated cylinder, measure out 4.5 ml of distilled water and pour it into a clean test tube. 5. From your 1 ml of 0.1M HCl remaining in your small beaker carefully pipette 0.5 ml into the test tube with your 4.5 ml of water. 6. Put 2 ml of this solution into two new test tubes. This will be concentration 2 set 1 and 2. SAFE WORK PROCEDURE Determining Hydrogen Ion Concentration of Strong and Weak Acids Page 2 of 2 7. Label these test tubes as having a concentration of 0.01M HCl. 8. Save the remaining 1 ml of 0.01M HCl for making concentration 3. 9. Using a 10 ml graduated cylinder measure out 4.5 ml of distilled water and pour it into a clean test tube. 10. From your 1 ml of 0.01M HCl remaining pipette 0.5 ml into the test tube with your 4.5 ml of water. 11. Put 2 ml of this solution into two new test tubes. This will be concentration 3 set 1 and 2. 12. Label these as having a concentration of 0.001M HCl. 13. Save the remaining 1 ml of 0.001M HCl for concentration 4. 14. Measure out 4.5 ml of distilled water and put it into a clean test tube. 15. Pipette 0.5 ml of 0.001M HCl into your test tube. 16. Put 2 ml of this solution into two new test tubes. This will be concentration 4 set 1 and 2. 17. Label these as having a concentration of 0.0001M HCl. The remaining 1 ml can be discarded down the sink. 18. Add one drop of methyl orange to test tube in set 1. 19. Add one drop of orange IV indicator to each test tube in set 2. 20. These two sets of test tubes act as standard solutions because HCl is a strong acid. 21. Obtain 5 ml of the 1.0M concentration of acetic acid. 22. Put it in your rinsed small beaker. Place 2 ml of the solution into two new test tubes. 23. Add one drop of methyl orange to test tube one and one drop of orange IV to test tube 2. 24. Compare the colours of these solutions to your standard solutions to determine the concentration of hydrogen ions. 25. Clean up your workstation. REGULATORY REQUIREMENTS WS&H Act W210, Section 4, 5 Mb. Regulations 217/2006, Part 16, (Machines / Tools & Robots) Sections 16.1-16.18) SAFE WORK PROCEDURE Part 35, (WHMIS Application) Part 36, (Chemical & Biological Substances Application) Determining Hydrogen Ion Concentration of Strong and Weak Acids Page 3 of 2