A novel role for XIST in modulating gender specific inflammatory
advertisement

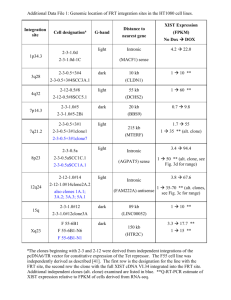
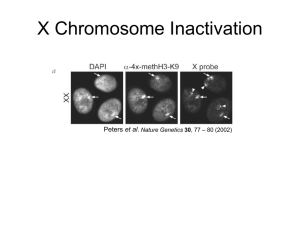
A novel role for XIST in modulating gender specific inflammatory response Botros Shenoda1, Guillermo Alexander2, Enrique Aradillas Lopez2 and Seena Ajit1 1 Pharmacology & Physiology, 2Neurology, Drexel University College of Medicine, Philadelphia, PA 19102 Introduction: Inflammatory and immune responses differ between the sexes. The levels of inflammatory cytokines also vary between males and females. X-chromosome inactivation is a complex epigenetic phenomenon and XIST (X-inactive specific transcript), a long non-coding RNA is crucial for mediating X-chromosome inactivation in female cells. Here we propose that female specific XIST expression may play a role in mediating sexual dimorphism in inflammatory response. Methods: To understand the role of XIST in mediating inflammatory response, we altered the expression of Xist under inflammatory stimuli using molecular and pharmacological tools. We investigated XIST expression in whole blood as well as sorted immune cells from patients with complex regional pain syndrome (CRPS) a chronic inflammatory and neuropathic pain condition. Results: Our results have shown that the expression of Xist in murine female macrophages is upregulated under inflammatory states through NF-κB mediated pathway, concomitantly decreasing proinflamamtory cytokines. XIST was also regulated by anti-inflammatory glucocorticoids, beta estradiol, and miR-34a and miR-181a, two microRNAs involved in mediating inflammatory response. Immune cells isolated from CRPS patients show reduced XIST levels relative to control while circulating XIST was significantly elevated in female CRPS patients relative to healthy controls. High circulating levels of XIST were also observed in female CRPS patients resistant to intravenous ketamine treatment. Conclusion: Collectively, these data indicate that miRNA mediated regulation of XIST in female cells may regulate inflammation associated with CRPS, and this miRNA-XIST-inflammation axis is modulated by beta estradiol. Our results reveal a novel function for XIST beyond X-chromosome inactivation. By suppressing excessive inflammatory response, XIST may contribute to lower levels of inflammatory cytokines in female patients relative to males. Fund: Botros Shenoda is a recipient Fulbright grant funded by the US department of the state and Dean’s Fellowship for Excellence in Collaborative or Themed Research from Drexel College of Medicine. This study was supported by funds from NINDS 1R21NS082991, Rita Allen Foundation and Drexel University Clinical and Translational Research Institute award to Seena Ajit.