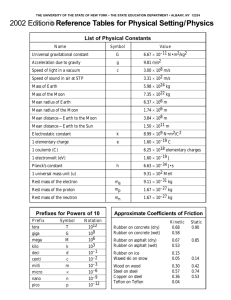
c = λf E= hf c = 3.00 x 108 m h = 6.6262 x 10
advertisement
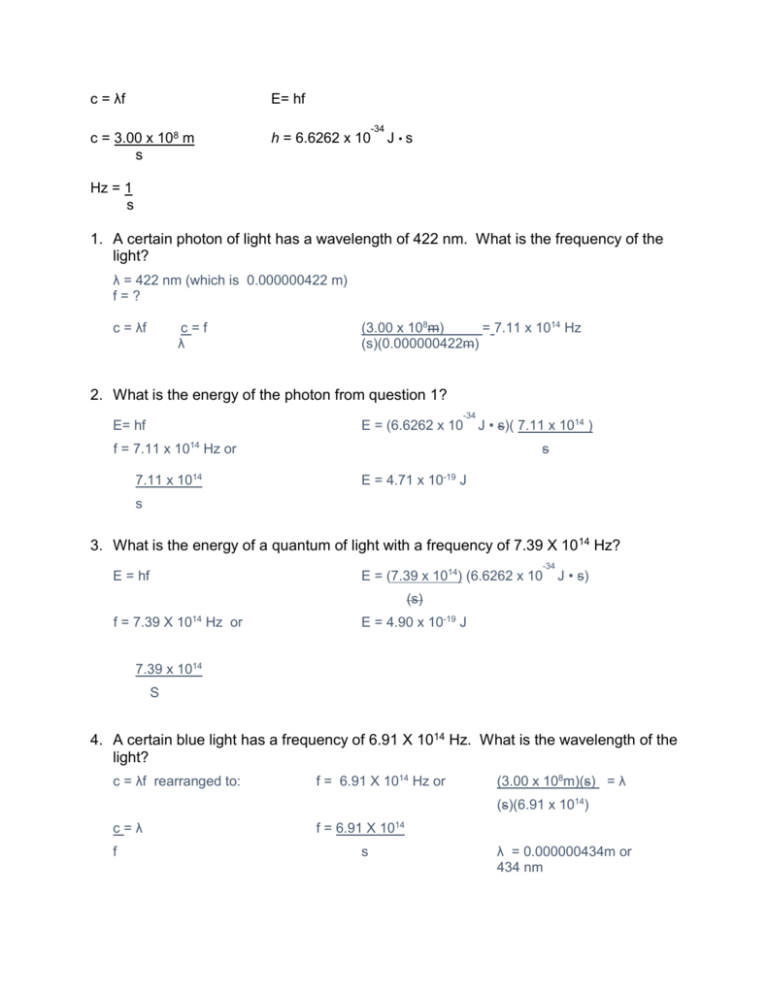
c = λf E= hf c = 3.00 x 108 m s h = 6.6262 x 10 -34 J •s Hz = 1 s 1. A certain photon of light has a wavelength of 422 nm. What is the frequency of the light? λ = 422 nm (which is 0.000000422 m) f=? c = λf c=f λ (3.00 x 108m) = 7.11 x 1014 Hz (s)(0.000000422m) 2. What is the energy of the photon from question 1? -34 E= hf E = (6.6262 x 10 f = 7.11 x 1014 Hz or 7.11 x 1014 J • s)( 7.11 x 1014 ) s E = 4.71 x 10-19 J s 3. What is the energy of a quantum of light with a frequency of 7.39 X 10 14 Hz? -34 E = (7.39 x 1014) (6.6262 x 10 E = hf J • s) (s) f = 7.39 X 1014 Hz or E = 4.90 x 10-19 J 7.39 x 1014 S 4. A certain blue light has a frequency of 6.91 X 1014 Hz. What is the wavelength of the light? c = λf rearranged to: f = 6.91 X 1014 Hz or (3.00 x 108m)(s) = λ (s)(6.91 x 1014) c=λ f f = 6.91 X 1014 s λ = 0.000000434m or 434 nm 5. The energy of a quantum of light is 2.84 X 10-19J. What is the frequency of the light? E = hf E= 2.84 X 10-19J rearranged to: (2.84 X 10-19J) =f (6.6262 x 10.34 J• s) E=f f = 4.29 x 1014 H s or 4.29 x 1014 Hz 6. Write the alpha decay equation for the following elements: U→ 238 4 92 2 He + Cm → 4 He + 243Pu 234 247 90 96 Th 2 94 7. Write the beta decay equation for the following elements: U→ 238 238 92 93 0 Np + Cm → e -1 247 247 Bk + 0 e 96 97 -1 8. Write the gamma decay equation for the following elements: U* → U+ 0ᵞ 238 238 92 92 0 Cm* → Cm + 0 ᵞ 247 247 96 96 0 9. What is the difference between fission and fusion? Fission splits a large, heavy nucleus (like Uranium) into two smaller, lighter nuclei. Fusion combines two small, light nuclei (like hydrogen), into a larger, more stable nucleus (like helium). 10. What are the three types of radiation/decay? Describe what happens to cause each type. Alpha radiation: Caused when the nucleus contains too many protons and the nuclear forces that hold them together “break”. A helium atom is expelled during the decay process. Beta radiation: Caused when the number of neutrons is too high compared to the number of protons. The atom converts a neutron into a proton and an electron. The proton stays in the atom, but the electron is expelled. Gamma radiation: Caused when an atom has too much energy and has become unstable. As it lowers back into a stable state of energy, photons of energy known as gamma rays are emitted. These are not particles that have mass, they are pure energy. 11. Be able to define wavelength, amplitude, frequency, crest, trough, origin (of a wave). Be able to pick out the correct diagrams if given multiple choice. Wavelength = the length of one complete wave Amplitude = the distance from the origin to the top of a crest or bottom of a trough Frequency = the number of waves that pass a certain point in a given amount of time. It is measure in Hz (hertz), or 1/s. Crest = a peak of a wave Trough = a valley of a wave Origin = the center line drawn directly through the middle of the wave. 12. Describe Planck and Einstein’s contributions to wave and particle science. Planck developed the quantum theory and planck’s constant. Einstein developed the Wave-Particle Duality theory, discovered the photoelectric effect, and developed the formula for deriving energy from the change in mass and speed of light (e = mc2). 13. What is a photon? A photon is a particle of light that carries a quantum of energy. 14. Be able to explain why the energy release from a nuclear change is so great. *hint: E = mc2 slide. Because the value for c2 is so large, even a tiny amount of mass defect multiplied by such a large number would result in an enormous energy release. 15. Be able to label a diagram of a nuclear powerplant. 16. Order the following in terms of lowest to greatest energy release: fission, coal fired power, fusion. Coal fired power, fission, fusion 17. Be able to determine the color of a given wavelength of light (memorize the wavelengths of the main light colors). 18. Be able to list the following waves in order of energy: Gamma rays, x rays, UV waves, Visible light waves, Infrared waves, microwave waves, radio waves This order was highest to lowest energy 19. Know how wavelength, frequency, and energy relate to each other: Wavelength and frequency are inversely proportional. Energy and frequency are directly proportional.