Supplemental Content - JACC: Clinical Electrophysiology
advertisement

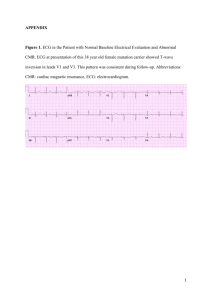
Supplementary Material Supplementary Table 1. Definitions of clinical and ECG variables employed in this study. Term Definition Sudden cardiac death Death of cardiac origin that occurred unexpectedly within 1 hour of the onset of new symptoms or a death that was unwitnessed and unexpected An event as described above, that is reversed, usually by cardio-pulmonary resuscitation and/or defibrillation or cardioversion Ventricular tachycardia which lasts 30 s or more, or less than 30 s when terminated electrically or pharmacologically ICD shock or anti-tachycardia overdrive pacing delivered in response to a ventricular tachyarrhythmia and documented by stored intracardiac ECG data Distinct waves of small amplitude within the ST segment in the right precordial leads and are distinct from the QRS complex The longest value in V1–V3, from the nadir of the S wave to the end of all depolarization deflections. Considered abnormal when ≥55 msec QRS duration ≥0.12 s, secondary R wave in right precordial leads, and wide S wave in leads I and V6 Sudden cardiac arrest Sustained ventricular tachycardia Appropriate ICD Therapy Epsilon wave Terminal activation duration Complete right bundle branch block Proven or definite ARVD/C (Diagnostic terminology as per 2010 revised Task Force Criteria) 2 major or 1 major and 2 minor criteria or 4 minor from different categories Heart failure Stage C heart failure was defined using the American College of Cardiology/American Heart Association heart failure staging system, but required both evidence of structural heart disease including RV abnormalities and symptoms directly attributed to heart failure. Nonsense, frame shift, splice site mutations and exon deletions were all considered to be proven pathogenic mutations unless previously identified as polymorphism or non-pathogenic. Missense mutations were considered pathogenic when: 1) the minor allele frequency in the Exome Sequencing Project (ESP) dataset was ≤0.05% (NHLBI 6500 Exome data sets; EVS; http://evs.gs.washington.edu/EVS/) and 2) in silico predictive programs (SIFT) and Polymorphism Phenotyping-2 (PolyPhen-2) both predicted the genetic variants to affect protein function by a tolerance index score of <0.02 (SIFT) and >0.900 (PolyPhen-2), respectively. The proband (index patient) was the first affected family member seeking medical attention for ARVD/C in whom the diagnosis was confirmed (i.e. an affected person ascertained independently of family history of ARVD/C and in whom DNA analysis was started) Pathogenic Mutation Proband (index patient) Family members Individuals ascertained through family screening. Abbreviations: ICD= Implantable Cardioverter-Defibrillator; VT= Ventricular tachycardia; VF= Ventricular fibrillation; ARVD/C= Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy Supplementary Table 2. List of 322 mutations identified in the study population. Gene; n (%) Amino Acid Change Nucleotide Change PKP2 Abnormal splice product p.(Arg79*) p.(Val406fs) p.(Trp538*) p.(His733fs) Abnormal splice product Abnormal splice product p.(Cys796Arg) p.(Gly548fs) p.(Thr50fs) Abnormal splice product p.(Gln133*) p.(Asn456fs) p.(Ser837fs) p.(Arg413*) Abnormal splice product Abnormal splice product c.2146-1G>C c.235C>T c.1211-1212insT c.1613G>A c.2197_2202delinsG c.2489+1G>A c.1171-2A>G c.2386T>C c.1643delG c.148_151delACAG c.2489+4A>C c.397C>T c.1368delA c.2509delA c.1237C>T c.337-2A>T c.224-3C>G Deletion exon 1-14 c.1821dupT c.1767T>G c.1307_1315insATTTA c.390C>T c.2145+1G>A c.2013delC c.1271T>C c.1369_1372delCAAA c.368G>C c.1759delG c.218dupG c.1132C>T c.968_971delAGGC c.1848C>A c.1849C>T c.2169_2172dupAGTT c.2174T>A c.253_256delGAGT c.517C>T c.663C>A c.1378+1G>C c.1760delT c.2544G>A c.1844C>T c.451delT c.14delG p.(Val608fs) p.(Tyr589*) p.(His436fs) p.(Tyr130*) Abnormal splice product p.(Lys672fs) p.(Phe424Ser) p.(Gln457*) p.(Trp123*) p.(Val587fs) p.(Asn74fs) p.(Gln378*) p.(Gln323fs) p.(Tyr616*) p.(Gln617*) p.(Val725fs) p.(Val725Asp) p.(Glu85fs) p.(Gln173*) p.(Tyr221*) Abnormal splice product p.(Val587fs) p.(Trp848*) p.(Ser615Phe) p.(Ser151fs) p.(Gly5fs) Pediatric n 5 1 3 2 5 5 0 1 0 1 1 0 1 1 1 1 1 0 1 1 0 1 0 0 1 0 0 1 2 1 1 0 1 0 0 1 0 1 1 0 1 0 0 0 Adult n 28 21 10 9 17 16 2 4 2 13 4 4 0 8 4 1 0 5 1 0 1 0 1 5 2 3 1 0 0 3 1 1 4 2 1 2 1 2 3 3 1 1 1 1 p.(Glu601fs) p.(Lys258*) p.(Arg651*) p.(Asn480fs) p.(Asn759fs) p.(Pro716fs) DSG2 PLN DSP DSC2 JUP TMEM43 Compound/ Digenic Heterozygote p.(Arg292Cys) p.(Arg46Gln) p.(Arg46Trp) p.(Cys507Tyr) p.(Glu156fs) p.(Gln812Cys) p.(Asp297Asn) p.(Arg49His) p.(Tyr1047Arg) p.(Lys346del) p.(Arg14del) p.(Arg2160*) p.(Arg2166*) p.(Arg160*) p.(Arg1738*) p.(Gln51*) p.(Glu422Lys) p.(Ile445Val) p.(Lys1054fs) p.(Gly220Arg) p.(Gly863fs) Abnormal splice product p.(Thr275Lys) p.(Thr19Ile) p.(Val159Leu) p.(Ser358Leu) p.(Val406fs) (PKP2) + p.(Gln90Arg) (DSP) p.(Gln133*) (PKP2) + p.(Thr872Ile) (PKP2) p.(Ala358Thr) (DSG2) p.(Glu426Lys) (DSC2) + p.(Pro1565Ala) (RYR2) p.(Arg203His) (DSC2) p.(Arg2160*) (DSP) + p.(Arg315Cys) (DSP) c.1803delC c.722A>T c.1951C>T c.1440_1444delTCCCA c.2274delG c.2146-1G>C Deletion exon 10 Deletion exon 7-14 Deletion exon 1-4 c.874C>T c.137G>A c.136C>T c.1520G>A c.464_465insT c.2434G>T c.889G>A c.146G>A c.3140C>G c.1038_1040delGAA c.40_42delAGA c.6478C>T c.6496C>T c.478C>T c.5212C>T c.151C>T c.1264G>A c.1333A>G c.3160_3169del c.658G>A c.2582_2585dupGAAG c.154+G>A c.824C>A c.56C>T c.475G>T c.1073C>T 0 0 0 0 1 0 0 1 0 0 3 1 0 0 0 0 0 0 0 0 0 0 0 1 0 1 0 0 1 0 1 1 1 0 0 1 1 3 1 0 1 2 0 2 2 2 0 1 1 2 1 2 1 1 23 1 2 2 1 1 0 1 1 0 1 0 0 0 1 1 c.1211_1212insT (PKP2) + c.269A>G (DSP) c.397C>T (PKP2) + c.2615C>T (PKP2) Deletion exon 1-14 (PKP2) + c.1072G>A (DSG2) c.1276G>A (DSC2) + c.4693C>G (RYR2) c.608G>A homozygosity (DSC2) c.6478C>T (DSP) + c.943C>T (DSP) 0 1 0 5 1 0 0 1 1 0 0 1 Abnormal splice product (PKP2) Mutant splice product (PKP2) + p.(Asn661Ile) (DSP) p.(Arg413*) (PKP2) + p.(Leu277_Met280del) (DSG2) p.(Lys346del) (DSG2) + p.(Ala358Thr) (DSG2) Abnormal splice product (DSG2) + (Lys346del) (DSG2) p.(Arg79*) (PKP2) + p.(Phe829fs) (PKP2) p.(Trp306*) (DSG2) + p.(Arg49His) (DSG2) p.(Glu2313fs) (DSP) + p.(Ser800Cys) (DSG2) c.2484C>T homozygosity (PKP2) c.2489+4A>C (PKP2) + c.1982A>T (DSP) 1 0 0 1 c.1237C>T (PKP2) + c.824_840del (DSG2) 0 1 c.1038_1040delGAA (DSG2) + c.1072G>A (DSG2) c.523+2T>C (DSG2) + c.1038_1040delGAA (DSG2) 1 0 1 0 c.235C>T (PKP2), c.2487delT (PKP2) c.918G>A (DSG2) + c.146G>A (DSG2) c.6937delG (DSP) + c.2399C>G (DSG2) 0 2 0 1 0 1 Abbreviations: DSC2: desmocollin-2; DSG2: desmoglein-2; DSP: desmoplakin; JUP: plakoglobin; PKP2: plakophilin-2; PLN: phospholamban; RYR2: Ryanodine Receptor-2; TMEM43: transmembrane protein 43. Supplementary Table 3. Demographic Details of 11 Children Who Presented With A Sudden Death. For the purpose of this study, the cause of death was defined as ARVD/C only if children were confirmed to harbor a pathogenic ARVD/C-causing mutation, or had a positive family history of ARVD/C. Single sudden death cases with questionable autopsy findings were excluded. Sex Age at SCD Family status Circumstance of SCD Prior medical evaluation Mutation* Autopsy findings Symptoms prior to SCD 1 Female 15 Proband No PKP2; p.Q74fsX84 No records None 2 Male 17 Proband Playing basketball Playing hockey No PKP2; p.A733fsX740 “Chest discomfort” 3 Male 16 Proband No PKP2; p.IVS10-1G>C 4 Male 16 Proband Playing basketball Playing basketball No PKP2; p.IVS12+1G>A 5 Female 13 Proband Rest No None 6 Male 15 Hunting No PKP2, p.A733fxX740 7 Male 16 PKP2; p.Q74fsX84 No records None Female 14 Getting up to go to bathroom Playing basketball No 8 Family member (brother of proband) Family member (brother of proband) Family member (sister of proband) Biventricular hypertrophy, fibrofatty RV replacement, aneurysm RV free wall Biventricular dilatation with fatty infiltration RV>LV Macroscopic fatty infiltration of basal anterior and inferior RV and posterolateral LV Fatty infiltration of RV subepicardial until endocardium, with areas of fibrosis and strands of normal heart tissue Inflammatory changes in RV No PKP2: mutant splice product No records 9 Female 16 In bed No None RV dilatation with wall thinning and fibrofatty replacement No records 10 Male 14 Playing soccer No PKP2; p.IVS12+1G>A No records 11 Male 17 Fibrofatty RV (and to lesser extent, LV) infiltration with focal absence of myocardium No records Family member (sister of proband) Family member (son of proband) No records No records None No records None Family member Gym class No PKP2; p.V406SfsX4 Presyncope (brother of proband) during exercise * SCD cases who did not have genotype studies were assumed to have the same mutation as other genetically affected first-degree relatives of their respective family. Supplementary Table 4. Ascertainment of pediatric and adult ARVD/C subjects, and fulfillment of 2010 Task Force Criteria in the “family history” category. Pediatric and adult ARVD/C cases are ascertained similarly, resulting in a similar proportion of probands and family members in the two groups. In addition, pediatric ARVD/C cases do not rely on family history criteria more often than adults. Abbreviations as in text. Ascertainment Relative coming to attention by family screening 2010 TFC for Family History Mutation carrier 1st degree affected relative per TFC 1st degree affected relative autopsy Possible ARVD/C in 1st degree relative but impossible to determine TFC Premature SCD <35yo due to suspected ARVD/C 2010 TFC without family history Relying on Family History criteria for definite diagnosis Overall (n=468) Pediatric (n=64) Adult (n=404) P-value 139 (30) 14 (22) 125 (31) 0.140 275 (59) 142 (31) 40 (9) 23 (5) 51 (80) 17 (26) 3 (5) 3 (5) 224 (55) 125 (31) 37 (9) 20 (5) <0.001 0.457 0.229 0.918 38 (8) 3 (5) 35 (9) 0.273 5 (IQR 4-6) 94 (20) 5 (IQR 4-6) 12 (19) 5 (IQR 4-6) 82 (21) 0.242 0.776 Supplementary Table 5. Clinical phenotype and outcome among 346 probands. Abbreviations as in text. Overall (n=346) Male 207 (60) Age at presentation (yrs) 34.8 ± 14.8 Proband 346 (100) Mutation carrier 197 (57) Clinical phenotype and Disease Course* Duration of follow-up (yrs) 8.9 ± 7.9 Repolarization criteria 302 (92) Negative T-wave V1-3 269 (82) Negative T-wave V1-2 16 (5) Negative T-wave V4-6 10 (3) Negative T-wave V1-4 w/ CRBBB 26 (8) Depolarization criteria 207 (64) Epsilon wave 42 (13) >TAD 140 (43) Late potentials 124/273 (45) Arrhythmia criteria 309 (94) LBBB superior axis VT 169 (52) LBBB VT 233 (71) Holter monitor >500 PVC/24hrs 195/243 (80) Pediatric (n=55) 32 (58) 15.5 ± 1.9 55 (100) 43 (78) Adult (n=291) 175 (60) 38.4 ± 13.2 291 (100) 154 (53) P-value 9.8 ± 8.6 48 (96) 45 (90) 2 (4) 1 (2) 4 (8) 30 (60) 6 (12) 21 (42) 15/42 (36) 265 (95) 24 (48) 34 (68) 35/37 (95) 8.7 ± 7.8 254 (92) 224 (81) 14 (5) 9 (3) 22 (8) 177 (64) 36 (13) 119 (43) 109/231 (47) 44 (88) 145 (52) 199 (72) 160/206 (78) 0.565 0.292 0.120 0.750 0.637 0.989 0.577 0.832 0.851 0.170 0.041 0.588 0.607 0.017 3015 5027 2587 (IQR 1003-7185) (IQR 2347-10907) (IQR 951-6444) Imaging abnormal 261 (80) 44 (88) 217 (79) Major 230 (71) 40 (80) 190 (69) Minor 31 (10) 4 (8) 27 (10) Major adverse clinical event 279 (85) 40 (80) 239 (86) Life-threatening arrhythmia 308 (66) 39 (78) 239 (86) Cardiac transplantation 18 (6) 2 (4) 16 (6) Cardiac death 18 (6) 4 (8) 14 (5) * Among 329 probands presenting alive. 0.001 PVC count/24hrs 0.786 <0.001 <0.001 0.129 0.304 0.168 0.619 0.393 Supplementary Table 6. Clinical phenotype and outcome among 156 family members. Abbreviations as in text. Overall (n=156) Male 57 (37) Age at presentation (yrs) 35.7 ± 15.3 Proband 0 (0) Mutation carrier 125 (80) Clinical phenotype and Disease Course* Duration of follow-up (yrs) 7.1 ± 6.3 Repolarization criteria 112 (82) Negative T-wave V1-3 80 (58) Negative T-wave V1-2 20 (15) Negative T-wave V4-6 9 (7) Negative T-wave V1-4 w/ CRBBB 4 (3) Depolarization criteria 75 (55) Epsilon wave 8 (6) >TAD 46 (34) Late potentials 47/107 (44) Arrhythmia criteria 92 (67) LBBB superior axis VT 14 (10) LBBB VT 31 (23) Holter monitor >500 PVC/24hrs 77/123 (63) PVC count/24hrs 1017 (IQR 181-3843) Imaging abnormal 78/136 (57) Major 45 (33) Minor 33 (24) Major adverse clinical event 30 (22) Life-threatening arrhythmia 30 (22) Cardiac transplantation 1 (1) Cardiac death 1 (1) * Among 139 family members presenting alive. Pediatric (n=20) 9 (45) 14.9 ± 3.4 0 (0) 17 (85) Adult (n=136) 48 (35) 38.8 ± 13.9 0 (0) 108 (79) P-value 6.3 ± 5.9 10 (71) 8 (57) 2 (14) 0 (0) 0 (0) 9 (64) 0 (0) 5 (36) 5/12 (42) 7 (50) 0 (0) 0 (0) 7/14 (50) 7.2 ± 6.3 102 (83) 72 (59) 18 (15) 9 (7) 4 (3) 66 (54) 8 (7) 41 (33) 42/95 (44) 85 (69) 14 (11) 31 (25) 70/109 (64) 0.513 0.291 0.920 0.972 0.295 0.493 0.449 0.325 0.858 0.867 0.163 0.185 0.034 0.301 1473 (IQR 204-4594) 8/14 (57) 5 (36) 3 (21) 1 (7) 1 (7) 0 (0) 0 (0) 1006 (IQR 178-3784) 70/122 (57) 40 (33) 30 (25) 29 (23) 29 (23) 1 (1) 1 (1) 0.763 0.400 <0.001 0.559 0.987 0.166 0.166 0.737 0.737 Supplementary Table 7. Clinical phenotype and outcome in pediatric ARVD/C vs. adult ARVD/C corrected for proband status. Odds ratios did not change >10% in multivariate analyses correcting for proband status, justifying a combination of proband and family members in the manuscript. Abbreviations as in text. Odds ratio* Male 1.06 (9.64-1.75) Mutation carrier 2.72 (1.48-5.00) Clinical phenotype and Disease Course Duration of follow-up (yrs) 1.01 (0.98-1.05) Repolarization criteria 1.08 (0.44-2.69) Negative T-wave V1-3 1.54 (0.76-3.12) Negative T-wave V1-2 0.87 (0.29-2.57) Negative T-wave V4-6 0.36 (0.05-2.78) Negative T-wave V1-4 w/ CRBBB 0.89 (0.30-2.65) Depolarization criteria 0.97 (0.56-1.67) Epsilon wave 0.78 (0.32-1.92) >TAD 0.98 (0.57-1.68) Late potentials 0.68 (0.37-1.23) Arrhythmia criteria 0.40 (0.19-0.86) LBBB superior axis VT 0.76 (0.43-1.36) LBBB VT 0.66 (0.37-1.18) Holter monitor >500 PVC/24hrs 1.64 (0.76-3.56) Imaging abnormal 1.54 (0.78-3.04) Major adverse clinical event 0.58 (0.29-1.13) Life-threatening arrhythmia 0.53 (0.27-1.02) Cardiac transplantation 0.66 (0.15-2.93) Cardiac death 1.56 (0.50-4.89) p-value 0.827 0.001 0.538 0.864 0.227 0.795 0.329 0.836 0.909 0.585 0.933 0.200 0.019 0.354 0.159 0.210 0.211 0.110 0.059 0.583 0.448 * Odds ratio denotes adult ARVD/C vs. pediatric (i.e. value >1 signifies higher odds in pediatric patients)