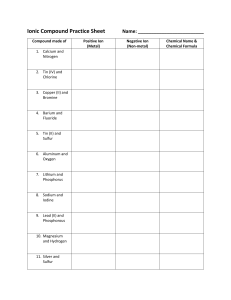
Unit 2 Test - Vocab/Concept List - Liberty Union High School District

Acid
Adhesion
Aerobic respiration
Ammonia
Ammonification
Atom
Atomic Mass
Atomic Number
Base
Biogeochemical cycle
Capillary action
Carbon Cycle
Carbohydrate
Closed system
Cohesion
Compound
Conservation of Mass
Covalent bond
Denitrification
Element
Energy
Unit 2 Test Review – Vocab and Concepts
Energy efficiency
First Law of Thermodynamics
Half-life
Hydrologic cycle
Inorganic compound
Input/output
Ion
Ionic bond
Isotope
Kinetic energy
Lipid
Macromolecules/biomolecules
Matter
Molecule
Negative Feedback Loop
Nitrates
Nitrification
Nitrites
Nitrogen Cycle
Nitrogen fixation
Nucleic acid
Nutrient cycle
Open system
Organic compound
Phosphate
Phosphorus cycle
Polar bond
Positive Feedback Loop
Potential energy
Protein
Proton/neutron/electron
Radioactive decay
Second Law of Thermodynamics
Steady state
Sulfur Cycle
Surface tension
Synergy
System analysis
Tipping point
Water Cycle
Work
1. What is the difference between low and high quality matter?
2. Energy doesn't recycle, but does matter?
3. What are the major steps to the carbon cycle, nitrogen, phosphorus, sulfur and water cycles?
4. What are the largest storage reservoirs for C, N, P and S? Which does not have an aerial form?
5. What specifically is upsetting the natural balance of the C, N, P and S cycles?
6. What are the main parts of the atom? How you determine the number of each subatomic particle in an atom?
7. What is carbon-dating and how does it relate to radioactivity? How do you calculate the half-life/use the half-life to predict amounts of radioactive samples?
8. What is energy? What is the difference between kinetic/potential energy?
9. What is energy efficiency?
10. What is a system? What’s the difference between open/closed systems? What is system analysis?
11. What are the 1 st and 2 nd laws of thermodynamics?
12. What’s the difference between positive and negative feedback loops?