CH 115 Fall 2014Worksheet 13 List the electronic geometry
advertisement

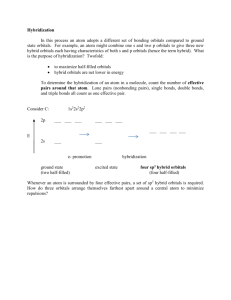
CH 115 Fall 2014 Worksheet 13 1. List the electronic geometry, molecular geometry, and bond angles for the central atom (or other specified atom) of the following molecules. a). COCl2 – trigonal planar, trigonal planar, 120o b). ClF4+ - trigonal bipyramidal, see-saw, less than 90o, 120o, and 180o c). SO2 – trigonal planar, bent, less than 120o d). HCOOH (carbon) – trigonal planar, trigonal planar, 120o e). IO4- - tetrahedral, tetrahedral, 190.5o Remember the first step to doing these problems is to draw a Lewis structure! 2. What is hybridization theory? What is a hybrid orbital? What is its purpose? (Think about why/how covalent bonds form). How do hybrid orbitals compare in energy to atomic orbitals? Hybridization is the process by which we mix atomic orbitals together to form hybrid orbitals that more accurately describe bonding patterns observed for certain molecules. Covalent bonds from through the overlap of these orbitals – hybrid orbitals better describe the geometry and angles we predict when we draw Lewis structures for compounds. Hybrid orbitals are intermediate in energy, or an average of the energies of the orbitals that were mixed to form them. 3. How do you determine the hybridization of an atom? What is the hybridization of each of the central atoms in question #1? 1. Draw the Lewis structure 2. Count the regions of electrons density around the atom in question 3. Assign hybridization (sp, sp2, sp3, sp3d, sp3d2) based on the number of regions Question # 1: a). sp2 b). sp3d c). sp2 d). sp2 e). sp3 4. What is a sigma bond? Which orbitals can be used to form it? In the most likely Lewis structure of SCN-, what two orbitals form the sigma bond between S and C? A sigma bond is the same thing as a single bond. It is formed when two orbitals overlap – those orbitals can be either atomic or hybrid orbitals depending on the hybridization of the atoms that make up the bond. Sigma bond between S and C is formed by sp3 and sp2 orbitals. 5. What is a pi bond? What orbitals can be used to form it? In the most likely structure of CO2, what two orbitals form the pi bond between O and C? A pi bond is a bond formed by the overlap ALWAYS of two unhybridized p (atomic) orbitals. Between O and C – 2p and 2p make the pi bond ALWAYS CH 115 Fall 2014 Worksheet 13 6. Fill in the table below. Single Bond Double Bond Triple Bond Sigma Bonds? 1 1 1 Pi Bonds? 0 1 2