Northern Ireland - Muscular Dystrophy UK
advertisement

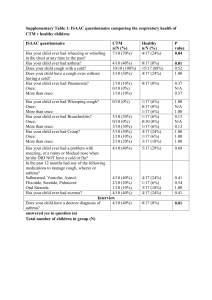
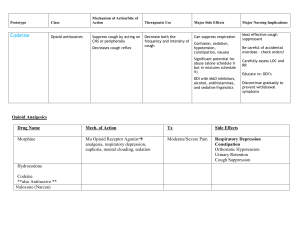
Dear MLA Name I am contacting you regarding access to cough assist machines for people affected by muscular dystrophy or a related condition (muscle-wasting conditions). Muscle-wasting conditions cause muscle to weaken and waste over time, leading to increased disability. This can affect muscles in the limbs, as well as the muscles of the heart and lungs, sometimes significantly shortening life-expectancy. With very few exceptions, there are currently no effective treatments or cures available. You could say something here about your own connection for muscular dystrophy or a related condition, and the impact on you and your family Some people affected by these conditions weakened cough means that individuals can find it very difficult to clear mucus from their chest and airways. This can lead to life threatening respiratory failure and an emergency admission to hospital. Evidence indicates that noninvasive breathing apparatus that assists with coughing (‘cough assists’) can effectively reduce the number of chest infections people get and help keep them out of hospital. Respiratory infections are the most common cause of hospital admissions1 and one of the primary causes of death for some types of neuromuscular condition. I was alarmed to learn from Muscular Dystrophy UK that individuals with muscle-wasting conditions across the UK are struggling to access these machines, due to the refusal of many local health commissioners to fund them. I am sure you will agree it is essential that people have access to the respiratory equipment they need, when they need it. I therefore write to ask if you will contact our local Trust to urge them to adopt a policy of funding cough assist machines if one is not already in place? For more information, please contact Peter Sutton at Muscular Dystrophy UK on 020 7803 4838 or email p.sutton@musculardystrophyuk.org Yours sincerely, 1 Bach JR, Ishikawa Y, Kim H. Prevention of pulmonary morbidity for patients with Duchenne muscular dystrophy. Chest 1997;112(4): 1024-1028 Commissioning Policy for Cough Assist Requests DOCUMENT CONTROL Reference Number Version Status Sponsor(s)/Author(s) (lead in specific policy Draft Version area to provide once 0.3 071015 policy ratified) Wendy Godwin Amendments Date By whom Approved by IFR panel 07/09/1 5 IFR Panel Members Approved Planned Care Programme Board 14/09/1 5 Programme Members Lead Commissioner Planned Care/Head of Elective Care Pathways Intended Recipients: Group/Persons Consulted: Head of Patient Safety and Quality Improvement Sally Roberts IFR team Board Robert Saunders and Dr Uma Viswanathan Planned Care Programme Board Monitoring Arrangements and Indicators: IFR database Training/Resource Implications: None CCG Value: Approving Body: Improving Outcomes Committee Ensure equity in access for all patients Date Approved: Date of Issue Review Date Contact for Review Lead Commissioner Planned Care/Head of Elective Care Pathways Policy Location: Intranet CCG Website Summary Evidence New evidence includes a systematic review, several RCTs, crossover trials, case series and retrospective cohort study, and overall the studies suggest that MI-E to assist cough is at least as effective as manual assisted cough. 2 RCTs found MI-E to be superior to other methods. N.B. Much of the evidence is for neuromuscular disease, but the clinical challenge is much the same in Spinal Cord Injury Explicit definition of patient group to which it applies (e.g. inclusion and exclusion criteria, diagnosis) Patients who have an ineffective/weak cough due to neuromuscular disease and cervical spinal cord injury. Specifically patients with conditions such as muscular dystrophy, spinal muscular atrophy, motor neurone disease and spinal cord injury Use of cough-assist machine is vital to enable expectoration of phlegm or mucus from throat or lungs, thus preventing A&E admission and emergency intubation. Respiratory function should be assessed in people with more complex care needs and consideration should be made of support from speech and language therapists and physiotherapist who as part of an MDT assessment can recommend appropriate interventions such as cough assist devices. The MDT may include palliative care and respiratory nurses to support people, for patients who require intensive interventions and cough assistance, and a rehabilitation consultation to advise on the best course of action when a significant worsening of symptoms occurs This commissioning policy describes the use of the cough assist machine to augment/assist an ineffective cough (determined by a reduced cough peak flow) in patients with neuro-muscular disease and spinal cord injury Abstract Contents Number Section Page No. 1.0 Introduction and Evidence 4 2.0 Implications 4 3.0 Prior Approval 5 4.0 Appendices 6 Commissioning Policy for Cough Assist Requests 1.0 Introduction and Evidence The mechanical insufflator/exsufflator (MI-E) assists the clearance of bronchopulmonary secretions in those patients with an ineffective cough by the use of both positive and negative pressure. Cough Assist is a non-invasive therapy that safely and consistently removes secretions in patients with an ineffective ability to cough (peak cough flow <270 l/m). The Cough Assist device clears secretions by gradually applying a positive pressure to the airway, then rapidly shifting to negative pressure. The rapid shift in pressure produces a high expiratory flow, simulating a natural cough. 1.1 Benefits of Cough Assist Removes secretions from the lungs Reduces the occurrence of respiratory infections Safe, non-invasive alternative to suctioning Easy for patients and caregivers to operate 1.2 Cough Assist Flexibility Can be used with a face mask, mouthpiece or with an adapter to a patient's endotracheal or tracheostomy tube Approved for home use in adults and children Available in automatic and manual models 1.3 Indications for Use 1.3.1 Typical Cough Assist patients include those with the following conditions: Amyotrophic lateral sclerosis Spinal muscular atrophy Muscular dystrophy Myasthenia gravis Spinal cord injuries Reduced Peak Cough Flow (PCF) of 160l/pm or 270 l/pm or < 270 l/pm and have clinical symptoms or a weak cough and therefor require intervention necessary to clear bronchial secretions or infection PCF can be measured by coughing into a peak flow meter attached to a mask MI-E Guidelines 2013 3 1.4 Contraindications Any patient with a history of bullous emphysema Susceptibility to pneumothorax or pnuemo-mediastinum Recent barotrauma, should be carefully considered before use The above contraindications should be carefully considered before use. 2.0 Implications Legal and/or Risk The risks of not providing this equipment outweighs the financial risks of making it available CQC N/A Patient Safety The Cough Assist Device piece can be required and may even be essential for the safe and timely discharge of spinal injury patient’s from an acute spinal bed into their own homes in the community. Patient Engagement BCNA representatives as Lay members are involved in the development of the policy as members of the Neurological Task and Finish Group Financial Reduction in spend on low priority treatments. The estimated cost for the Cough Assist equipment is £4,500 per patient with an additional £500 per year, on-going costs. Based on the current levels of demand the CCG would expect to have one patient every two years requiring the equipment. NHS England has commissioning responsibility for the acute treatment of spinal cord injuries. Their policy does however make it clear that responsibility passes back to CCGs once the patient is discharged from Acute care. The Cough Assist Device has been specifically mentioned as an item that CCGs may be required to provide for patients with suppressed cough reflex to support their discharge. NHS England policy also indicates that CCGs will be charged the cost of excess bed days resulting from delayed discharge if this equipment is not available. Protection of finance for essential (high priority) services Sustainability Workforce/Training The service provider will also arrange training on an ad-hoc basis. 3.0 Prior Approval This commissioning proforma covers the use Mechanical Insufflation-Exsufflation (MI-E) therapy for patients with neuromuscular disorders and cervical spinal cord injury patients 3.1 Clinical Indications for Funding 3.1.1. An established diagnosis as paralytic/restrictive disorder including but not exclusively: spinal cord injuries (SCI) neuromuscular diseases such as ALS Guillain-Barré Syndrome myasthenia gravis muscular dystrophy multiple sclerosis post polio kypho-scoliosis syringomyelia 3.2.2. Patient is unable to cough or clear secretions effectively with a PCF (Peak Cough Flow) less than 160 L/min VC (vital capacity) below 1.1L in general respiratory muscle weakness, or voluntary Reduced Peak Cough Flow (PCF) of 270 l/pm or < 270 l/pm and have clinical symptoms or a weak cough and therefor require intervention necessary to clear bronchial secretions or infection Requests for MI-E or 'cough assist therapy' for patients who do not meet the above criteria are considered low priority and will not be routinely funded. 3.2 Absolute Contra-Indications Presence of haemoptysis, untreated or recent pneumothorax, bullous emphysema, nausea and emesis, severe COPD, severe asthma and recent lobectomy Increased intra cranial pressure (ICP) including ventricular drains Impaired consciousness / inability to communicate in instances where the patient does NOT have an artificial airway 3.2.1 Relative Contraindications • therapy immediately following meals • tachypnea • history of COPD and pneumothorax • large pleural effusion • cervical spinal injury unclear • hemodynamic instability • impaired consciousness / inability to communicate where the patient has an artificial airway Supplemental oxygen should not be bled into the MI-E circuit. Oxygen passing through the fan system during the exsufflation phase results in a potential fire hazard Appendix 1 References 1. Motor Neurone Disease a Problem Solving Approach for General Practitioners and Allied Health Professionals 2011 http://www.mndscotland.org.uk/wpcontent/uploads/2011/08/A-Problem-Solving-Approach-2012.pdf 2. National Institute for Health and Care Excellence Multiple Sclerosis Stakeholder Comments – Draft Guideline June 2014 http://www.mssociety.org.uk/sites/default/files/Documents/Campaigns%20resources/MSSociety-response-to-draft-NICE-clinical-guideline-MS.pdf 3. NHS Evidence https://www.evidence.nhs.uk/search?q=cough+assist+machines 4. Nottingham University Hospital NHS Trust Cough Assist Guideline August 2013 5. Muscular Dystrophy UK 2015 #Right To Breath Campaign http://www.musculardystrophyuk.org/news/campaign-success-as-nhs-bosses-incornwall-agree-to-fund-cough-assist-machines/ Appendix 2 Evidence Base 1. Bach JR et al. Mechanical insufflation-exsufflation. Comparison of peak expiratory flows with manually assisted and unassisted coughing techniques.Chest. 1993 Nov;104(5):1553-62 2. Berlly M et al. Respiratory Management During the First Five Days After Spinal Cord Injury. J Spinal Cord Med. 2007; 30(4): 309–318 3. Chatwin M et al. Cough Augmentation with Mechanical Insufflation/Exsufflation in Patients with Neuromuscular Weakness. Eur Respir J: March 2003;21(3):502-508 4. LeBlanc C Asthma / COPD Educator Professional Practice Leader, Respiratory Therapy The Ottawa Hospital Rehabilitation Centre McKim Douglas A MD, FRCPC, FCCP, D,ABSM Medical Director, Respiratory Rehabilitation Services Associate Professor, Department of Medicine University of Ottawa 5. Reid WD et al. Physiotherapy Secretion Removal Techniques in People With Spinal Cord Injury: A Systematic Review. J Spinal Cord Med. 2010;33(4):353–370 6. Respiratory Therapy Policy And Procedure Mechanical Insufflation-Exsufflation for Paralytic/Restrictive Disorders. 7. Sancho J et al. Mechanical in/exsufflation vs tracheal suctioning via tracheostomy tubes for patients with amyotrophic lateral sclerosis: a pilot study. Am J Phys Med Rehabil 2003;82(10)750-753 8. Tzeng AC & Bach JR. Prevention of Pulmonary Morbidity or Patients with Neuromuscular Disease. Chest 2000;118: 1390-1396 9. Winck JC et al. Effects of mechanical insufflation-exsufflation on respiratory parameters for patients with chronic airway secretion encumbrance. Chest. 2004;126:774-780