File - RHS Academic Chemistry
advertisement

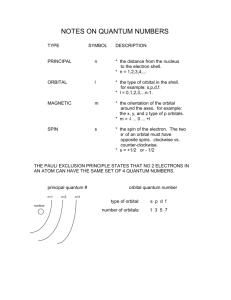
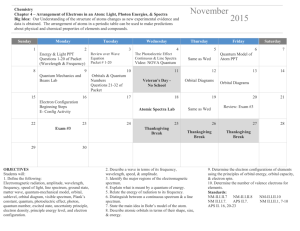
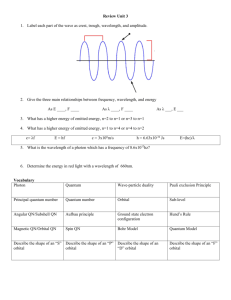
Name_________________________________________ Period_____ 4-2 / 4-3 Study Guide 1. What is the Heisenberg Uncertainty Principle? 2. Identify each of the following as s, p, d, or f orbitals. a. b. c. d. _____ orbital _____ orbital _____ orbital _____ orbital 3. Answer the following questions as true or false. If false, rewrite the statement to make it correct. a. The denser the region in a probability cloud, the less likely an electron would be found there. b. The probability cloud shows a specified volume of space where an electron would likely be found. 4. Draw the diagonal rule. (Note: You will be responsible for knowing this come quiz time.) 5. Write the orbital notation and electron configuration for the following elements. Element Orbital Notation Electron Configuration Niobium Iodine Lead Barium 6. Write the element that would end with the following configuration. a. 3p6____________________ b. 4p2____________________ c. 5d7____________________ d. 3d3____________________ e. 6s1____________________ f. 7. Match the three rules of electron configurations with their definition. ____a. Aufbau Principle i. ____b. Pauli-Exclusion Principle ii. ____c. Hund’s Rule iii. 4p6____________________ Orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron, and all of the electrons in singly occupied orbitals must have the same spin. An electron occupies the lowest energy orbital that can receive it. No two electrons in the same atom can have the same set of four quantum numbers. 8. Match the 4 quantum numbers with their descriptions. ____a. Principal Quantum Number i. Main energy level of an electron. ____b. Magnetic Quantum Number ii. Indicates the two fundamental spin states of an electron. ____c. Angular Momentum Quantum Number iii. Shows the orientation of an orbital around the nucleus. ____d. Spin Quantum Number iv. Indicates the shape of the atomic orbital of which the electron is located. 9. Draw the electron dot diagrams for the following elements. a. Gallium f. Rubidium b. Sulfur g. Chromium c. Strontium h. Fluorine d. Tantalum i. Silver e. Copper j. Lead 10. Use the patterns within the diagonal rule and the periodic table to draw the orbital diagrams and electron configurations for the following elements. Element Total Orbital Diagram Electron Configuration Number of Electrons Vanadium Germanium Krypton Lead Uranium* Cadmium Radon Gold* Cesium* Antimony 11. Which of the following “rules” is being violated in each electron configuration below? Explain your answer for each. A. B. C. D. __ __ 1s 2s 2p 1s 2s 1s 2s 1s 2s ___ _ _ 2p 3s 3p _ 2p 3s 3p 2p 3s 3p 3d