eILIProtocol - Public Health Surveillance
advertisement

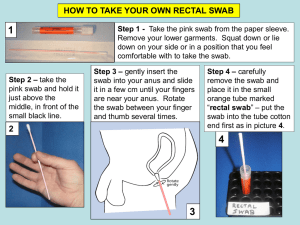
INSTRUCTIONS FOR PARTICIPATING GENERAL PRACTICES IN ELECTRONIC SENTINEL INFLUENZA SURVEILLANCE Case definition of influenza: An acute respiratory illness with a history of fever or measured fever of ≥38°C, AND cough, AND onset within the past 10 days When you identify cases of influenza like illness (ILI) each week, you are required to do the following: Open up the Health Link Form “New Zealand ILI Surveillance” , complete necessary information (See flow diagram Figure 1) Take a nasopharyngeal or throat swab* from one patient (preferably the first) who meets the case definition that you see on each Tuesday, and Wednesday and Thursday. (*Note: A nasopharyngeal swab is preferable since it contains more viral infected columnar epithelial cells than a throat swab. However, a throat swab is acceptable too.) Information to patients from whom nasopharyngeal or throat swabs are taken: We suggest that you explain the following to the patients who fit the case definition: that you would like to take a nasopharyngeal or throat swab that this is mainly for the purpose of designing next year's flu vaccine that the other advantage of taking the swab is that it may help confirm the diagnosis of flu for them as an individual and this service is free that they are welcome to refuse having a swab taken that they will be informed of the results Instructions on taking nasopharyngeal swab (NPS) 1. Label sample collection vial with patient name, NHI number (if known), date of birth, and date of collection. 2. Insert swab into one nostril, parallel to the palate, rotate gently and advance until resistance is felt (one eye often waters when swab is in correct position). 3. Press swab tip on the mucosal surface of the mid-inferior portion of the inferior turbinate (see diagram), leave in place for a few seconds, then slowly withdraw with a rotating motion. 4. Place tip of swab back into swab collection vial containing viral transport medium (VTM) and carefully break or cut the shaft of the swab. 5. Close the lid tightly. Instructions on taking throat swab 1. Label sample vial with patient name, NHI number (if known), date of birth and date of collection. 2. Get patient to say “ahhh” and vigorously swab both tonsillar areas and posterior nasopharynx. Use tongue depressor to depress tongue to prevent contamination of swab with saliva. 3. Place swab back into swab collection vial containing viral transport media and carefully break or cut the shaft of the swab. 4. Close the lid tightly. Supplying Viral Transport Materials The PHS co-ordinator should liaise with their designated virology laboratory about the need for viral transport medium (VTM), lab request forms, packaging etc and also arrange for the transportation of samples. They should ensure that all of the supplies are kept refrigerated. Transport of Specimens The PHS co-ordinator should ensure that swabs are despatched in an appropriate VTM, to the virus laboratory (see list overleaf). Specimens should arrive at the laboratory within three days of collection. To ensure minimal loss of viability of virus, it is important that the specimen is kept cold (4 to 8°C) from the time it is taken until arrival in the laboratory. Swabs in VTM should be sent by courier in a bio-bottle labelled "Influenza Surveillance" and with an ice pack (e.g. slikka pad) enclosed. ESR will provide courier tickets, bio bottle with outer cardboard box, vials containing viral transport medium, swabs, plastic specimen bags, plastic bubble bags, adsorbent pads, ice packs, specimen request forms and shipping and sampling instruction sheet. Specimens should be enclosed in the bio-bottle in the leak-proof bag contained, separate from forms in case of leakage. ESR will refund all the costs for any telephone calls (no collect calls, please) required for influenza surveillance. Delivery of the specimens Specimens should be sent to the ESR virology laboratory within three days of collection. Until despatch, swabs (and uninoculated bottles of VTM) SHOULD BE KEPT REFRIGERATED. Depending on the arrangement among sentinel GP practice, local influenza surveillance coordinator and the responsible virology laboratory, the specimens should be sent directly to ESR-National Centre for Biosecurity and Infectious Diseases. Virus Laboratory Address Phone number ESR-National Centre for Biosecurity and Infectious Diseases, Wallaceville, Upper Hutt (Director, Sue Huang) 66 Ward Street, Wallaceville, Upper Hutt 5018 (04) 529 0600 Key contacts at ESR are: Liza Lopez, Health Intelligence Team, at (04) 914 0647 Judy Bocacao, ESR-National Centre for Biosecurity and Infectious Diseases, at (04) 529 0618 Dr. Sue Huang, ESR-National Centre for Biosecurity and Infectious Diseases, at (04) 529 0606 To order viral transport materials please contact Amanda des Barres/Trish McCauley, Specimen Reception ESR-Wallaceville at (04) 529 0600. Patient consults GP Meets ILI Case Definition No Do Nothing No Open ILI form and fill out: Date of Encounter(defaults to today) Tick “Meets criteria for ILI” Select “No’ for Specimen collection Yes Meets Swab Definition SUBMIT Yes Open ILI form and fill out: Date of Encounter(defaults to today) Tick “Meets criteria for ILI” Select “Yes”for Specimen collection Patient consents to Swab No Select “No” to patient consents to swab SUBMIT Park form and inform Nurse Nurse gets ILI form for patient Yes Select “Yes” to patient consents to swab Is GP going to complete form No Yes Complete form SUBMIT Figure 1 Complete form