ina12061-sup-0001-AppS1-TableS1-FigS1-S2
advertisement

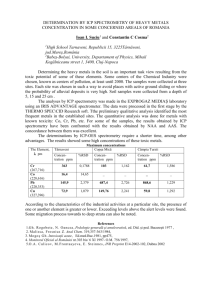
SUPPORTING INFORMATION APPLICATION OF PROTON-TRANSFER-REACTION-MASS-SPECTROMETRY (PTR-MS) FOR INDOOR AIR QUALITY RESEARCH Tobias Schripp1, Sebastian Etienne1, Christian Fauck1, Frank Fuhrmann1, Lukas Märk2, Tunga Salthammer1 1 Fraunhofer WKI, Department of Material Analysis and Indoor Chemistry Bienroder Weg 54E, 38108 Braunschweig, Germany 2 IONICON Analytik GmbH Eduard-Bodem-Gasse 3, 6020 Innsbruck, Austria PTR instruments in atmospheric sciences Due to the diversity of PTR technology there are various approaches for PTR-MS and PTRTOF systems. In some cases the inlet has been designed to analyze airborne particles in a multistep sampling and thermal desorption process (Winkler et al., 2013). The formation of proton ions may be performed by using a hallow cathode (Ennis et al., 2005), a direct current discharge source (Inomata et al., 2006), a radioactive source (Blake et al., 2004) or an ion funnel (Barber et al., 2012). With regard to the mass-filter the common quadrupol filter (Lindinger et al., 1998), an ion trap (Prazeller et al., 2003), a linear ion trap (Mielke et al., 2008) and a time-of-flight filter (Tanimoto et al., 2007) are used. The size of the components is also an important factor if the system is applied in flight (Wisthaler et al., 2013). Most of these instruments are not commercially available but are modifications of available instruments or self-developed systems. Barber, S., Blake, R.S., White, I.R., Monks, P.S., Reich, F., Mullock, S., Ellis, A.M. (2012) Increased Sensitivity in Proton Transfer Reaction Mass Spectrometry by Incorporation of a Radio Frequency Ion Funnel, Analytical Chemistry, 84, 5387−5391. Blake, R.S., Whyte, C., Hughes, C.O., Ellis, A.M. and Monks, P.S. (2004) "Demonstration of proton-transfer reaction time-of-flight mass spectrometry for real-time analysis of trace volatile organic compounds", Analytical Chemistry, 76, 3841-3845. Ennis, C.J., Reynolds, J.C., Keely, B.J. and Carpenter, L.J. (2005) "A hollow cathode proton transfer reaction time of flight mass spectrometer", International Journal of Mass Spectrometry, 247, 72-80. Inomata, S., Tanimoto, H., Aoki, N., Hirokawa, J. and Sadanaga, Y. (2006) "A novel discharge source of hydronium ions for proton transfer reaction ionization: design, characterization, and performance", Rapid Communications in Mass Spectrometry, 20, 1025-1029. Jordan, A., Haidacher, S., Hanel, G., Hartungen, E., Märk, L., Seehauser, H., Schottkowsky, R., Sulzer, P., Märk, T.D. (2009) “A high resolution and high sensitivity proton-transfer-reaction time-of-flight mass spectrometer (PTR-TOF-MS)”, International Journal of Mass Spectrometry, 286, 122-128. Lindinger, W., Hansel, A. and Jordan, A. (1998) "On-line monitoring of volatile organic compounds at pptv levels by means of proton-transfer-reaction mass spectrometry (PTR-MS) medical applications, food control and environmental research", International Journal of Mass Spectrometry and Ion Processes, 173, 191-241. Mielke, L.H., Erickson, D.E., McLuckey, S.A., Müller, M., Wisthaler, A., Hansel, A. and Shepson, P.B. (2008) "Development of a Proton-Transfer Reaction-Linear Ion Trap Mass Spectrometer for Quantitative Determination of Volatile Organic Compounds", Analytical Chemistry, 80, 8171-8177. Prazeller, P., Palmer, P.T., Boscaini, E., Jobson, T. and Alexander, M. (2003) "Proton transfer reaction ion trap mass spectrometer", Rapid Communications in Mass Spectrometry, 17, 1593-1599. Tanimoto, H., Aoki, N., Inomata, S., Hirokawa, J. and Sadanaga, Y. (2007) "Development of a PTR-TOFMS instrument for real-time measurements of volatile organic compounds in air", International Journal of Mass Spectrometry, 263, 1-11. Winkler, P.M., Lawler, M. and Smith, J.N. (2013) Size-resolved chemical characterization of biogenic nanoparticles by thermal desorption chemical ionization mass spectrometry, In: Hansel, A. and Dunkl, J. (eds) 6th International Conference on Proton Transfer Reaction Mass Spectrometry and its Applications, Obergurgl, Austria, Innsbruck University Press, 92-95. Wisthaler, A., Crawford, J.H., Haidacher, S., Hanel, G., Hartungen, E., Jordan, A., Märk, L., Mikoviny, T., Müller, M., Mutschlechner, P., Schottkowsky, R. and Sulzer, P. (2013) Development of a compact PTR-TOF-MS for Suborbital Research on the Earth's Atmospheric Composition, In: Hansel, A. and Dunkl, J. (eds) 6th International Conference on Proton Transfer Reaction Mass Spectrometry and its Applications, Obergurgl, Austria, Innsbruck University Press, 96. Additional Figures and Tables Normed Transmission 2.0 1.5 1.0 0.5 TOF-Transmission (actual) TOF-Transmission (ideal) Transmission curve (actual) Transmission curve (ideal) 0.0 0 200 400 600 800 1000 m/z Figure S1: Transmission curve of the applied PTR-TOF instrument in comparison to the expected transmission curve of a reference instrument (source: Ionicon). Table S1: Fitting parameters for a non-linear regression of the TEA-concentrations from Figure 3 using equation (S1). The exponential factors are given in bold. Paint 1 Paint 2 Paint 3 Paint 4 a 10.70 16.06 8.78 9.80 b 1.39 2.76 0.78 0.88 c 16.74 13.10 8.24 3.83 d 0.13 0.13 0.08 0.03 R 0.9998 0.9987 0.9966 0.9924 𝑐(𝑡) = 𝑎 ∙ (1 − 𝑒 −𝑏∙𝑡 ) + 𝑐 ∙ (1 − 𝑒 −𝑑∙𝑡 ) (S1) Counts (m/z x / m/z 21) 0.1 0.1 n-Pentanal (m) 0.1 Isobutyric acid (m) 0.1 0.01 0.01 0.01 0.01 0.001 0.001 56.6 56.8 0.1 Counts (m/z x / m/z 21) Acetic acid (m) n-Octanol (f)? 57.0 57.2 Hexanoic acid (f) 0.001 60.6 57.4 0.1 60.8 61.0 61.2 61.4 n-Hexanal (f) 0.001 86.6 0.1 86.8 87.0 87.2 87.4 Heptanoic acid (m) 88.6 0.1 0.01 0.01 0.01 0.01 0.001 0.001 0.001 0.001 98.6 98.8 99.0 99.2 99.4 100.6 100.8 101.0 101.2 101.4 130.6 130.8 131.0 131.2 131.4 Figure S2: Enlarged display of selected signals in Figure 7 for the PTR-QMS (red bar) and PTR-TOF (black line). 88.8 89.0 89.2 89.4 3-Caren (m) a-Pinen (m) 136.6 136.8 137.0 137.2 137.4