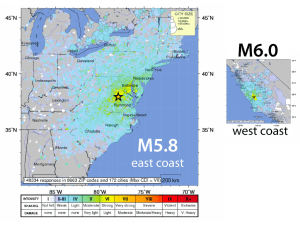
Crystal chemical constraints on inter-mineral Fe isotope fractionation
advertisement

1 APPENDIX A 2 3 1. Detailed sample description and background 4 The majority of the xenoliths in this study can be classified into one of two 5 groups, commonly referred to as Groups I and II (Frey and Prinz, 1978). Menzies (1983) 6 refined the classification but referred to Types I and II; in this paper we employ the 7 original terminology of Frey and Prinz (1978). Group I inclusions are mainly olivine-rich 8 peridotites, but pyroxene-rich lithologies are also present. The olivine-rich lithologies 9 include spinel lherzolite, spinel harzburgite, and spinel dunite. Pyroxenes and spinels in 10 Group I rocks are typically Cr-rich and TiO2 poor, and silicates generally have Mg 11 numbers (Mg#) >0.85. Bulk and mineral compositions and trace-element and isotope 12 geochemistry indicate that the olivine-rich Group I rocks are the products of extraction of 13 basaltic liquid during partial melting in the mantle lithosphere (Frey and Prinz, 1978). 14 Some of these rocks show trace-element and isotopic evidence, chiefly in clinopyroxenes, 15 of subsequent metasomatism by enriched magmas and/or CO2-H2O fluids (Frey and 16 Prinz, 1978; Menzies, 1983; Galer and O’Nions, 1989). Group I xenoliths are subdivided 17 into IA and IB to distinguish between those that respectively do not or do record evidence 18 for later metasomatism. Within IB xenoliths, the metasomatic signature is most 19 widespread in the most refractory, clinopyroxene poor samples. The pyroxene-rich 20 lithologies include orthopyroxenites, websterites, and clinopyroxenites with varying 21 olivine contents. The orthopyroxene-rich Group I rocks are interpreted as either residual 22 tectonic layers in peridotites, or as cumulates from the extracted basaltic liquid (Frey and 23 Prinz, 1978). Clinopyroxene-rich rocks may occur as layers or veins in lherzolites, or as 1 discrete xenoliths. They possess geochemical signatures of equilibration with alkaline 2 mafic magma. The common occurrence of exsolution lamellae in clinopyroxene implies 3 that this event predated entrainment in the basanitic magma that carried the xenoliths to 4 the surface (Frey and Prinz, 1978). 5 Group II xenoliths are highly variable in terms of mineral proportions and 6 compositions, and include spinel clinopyroxenites, spinel olivine websterites, and many 7 others. Kaersutitic amphibole is common. Pyroxenes in Group II xenoliths are usually 8 Al2O3- and TiO2-rich, but Cr-poor. This is reflected in Group II spinels and 9 clinopyroxenes, which are generally more Al-rich and Cr-poor than their Group I 10 counterparts. The Mg# of silicates ranges widely, but is typically <0.85. Group II 11 xenoliths are interpreted as cumulates from an SiO2-undersaturated magma, most likely 12 the host basanite (Frey and Prinz, 1978). Samples transitional between the two groups 13 have also been identified (Wilshire and Shervais, 1975; Wilshire and Jackson, 1975; Frey 14 and Prinz, 1978). 15 In this study, we focused on Group I xenoliths to assess Fe isotope systematics of 16 the local mantle lithosphere prior to modification by the magmatic event that brought 17 them to the surface. Five lithologically distinct xenoliths were studied, including spinel 18 lherzolite, spinel harzburgite, spinel dunite, clinopyroxenite, and olivine websterite. The 19 spinel lherzolite came from our private collection; the four other samples were obtained 20 from the Smithsonian Museum. 21 The mineral identities and textures in the Ol- and Cpx-rich xenoliths are 22 consistent with our classification of them as Group I (c.f., Frey and Prinz, 1978, their 23 table 1). The olivine websterite is more similar to Group II websterites studied by Frey 1 and Prinz, but it lacks amphibole and the pyroxene and spinel textures are more similar to 2 Group I samples. 3 Mineral compositional characteristics can also be used to distinguish Group I and 4 II samples (Table 1). Figure 3a shows Al2O3 content of pyroxenes vs. Cr number in 5 spinel. Using data from Frey and Prinz (1978) and Galer and O’Nions (1987), it can be 6 seen that these compositional parameters clearly distinguish xenoliths from Groups I and 7 II. Also shown are samples interpreted by Frey and Prinz to be Group I transitional. The 8 olivine- and clinopyroxene-rich xenoliths all lie in the Group I field. In contrast, the 9 olivine websterite appears to show transitional characteristics, in that the Cpx Al2O3 10 content is similar to the most aluminous Group I Cpx, but the spinel has low Cr# more 11 typical of Group II. Figure 3b shows a similar result: the Al2O3 contents of coexisting 12 Opx and spinel from Group I define a linear, positively correlated array. A transitional 13 Group I sample plots at the most aluminous end of this trend. In contrast, Group II 14 samples plot together at high but roughly constant Al2O3 spinel. Again, our olivine- and 15 clinopyroxene-rich samples lie along the Group I array. In contrast, the olivine websterite 16 sample displays characteristics of both groups, in that it lies along the array but is at the 17 highest Al2O3 contents of the data shown in the figure. 18 Thus, the clinopyroxenite and spinel-bearing lherzolite, harzburgite and dunite 19 possess characteristics that identify them clearly as Group I xenoliths. In contrast the 20 olivine websterite is more similar to xenoliths interpreted as transitional to Group II. In 21 the following, we refer to the websterite as transitional and treat it separately from the 22 other xenoliths. 1 The three peridotite xenoliths chosen for this study represent a wide range of 2 olivine abundance, including a spinel lherzolite (CEM1-3), a harzburgite (111-312-37), 3 and a dunite (111-312-26). Spinel lherzolite CEM1-3 is composed of 62% olivine (Ol), 4 24% orthopyroxene (Opx), 13% clinopyroxene (Cpx), and <1% chromian spinel (Spl). 5 The average spinel composition for this xenolith is 6 3+ VI IV (Mg 0.73 Fe2+ 0.27 ) (Al1.19 Cr0.74 Fe0.06 ) O4 as determined by charge balance assuming no 7 vacancies using the method described in Droop (1987). The spinels in this sample are 8 notable in having relatively high concentration of Cr as well as relatively small amounts 9 of Fe3+ assigned to the octahedrally coordinated site. Therefore, the iron in the spinel of 10 CEM1-3 is dominantly ferrous and tetrahedrally coordinated. Olivine in this sample is 11 Fo91 and contains negligible amounts of ferric iron (Fe3+/∑Fe = 0.04 ± 0.12), with all 12 iron being assigned to octahedrally coordinated sites. Iron in pyroxenes is also 13 octahedrally coordinated, although in most samples has higher ferric/ferrous ratios than 14 that of olivine. Clinopyroxene in CEM1-3 has Fe3+/∑Fe = 0.22 ± 0.08, and Opx has 15 Fe3+/∑Fe = 0.15 ± 0.03. BSE images of CEM1-3 reveal no petrographic signs of 16 metasomatism or interaction with a melt (Supp. Fig. 1a). Young et al. (2009) found that, 17 in this same xenolith, heavy Mg isotopes are concentrated in spinel compared to the other 18 minerals, and Mg isotope thermometry records a spinel-olivine equilibration temperature 19 of 815 ± 11 °C with no corrections for cation substitutions. 20 Sample 111-312-37 is a harzburgite consisting of 69% Ol, 27% Opx, 4% Cpx, 21 and <1% chromian Spl. The calculated average spinel formula is 22 3+ VI IV (Mg 0.72 Fe2+ 0.28 ) (Al1.04 Cr0.90 Fe0.05 ) O4 . These spinels also have high chromium content 23 and relatively low trivalent iron in octahedral coordination versus divalent iron in 1 tetrahedral coordination (Fe3+/∑Fe = 0.16 ± 0.01). Olivine, Cpx, and Opx have Fe3+/∑Fe 2 of 0.07 ± 0.08, 0.21 ± 0.54, and 0.12 ± 0.06, respectively. BSE images of this harzburgite 3 show partially altered regions near the basalt-xenolith contact (Supp. Fig. 1b). 4 Sample 111-312-26 is a dunite with 69% Ol, 27% Opx, 4% Cpx, and <1% Spl. 5 3+ VI IV The average spinel formula is (Mg 0.79 Fe2+ 0.21 ) (Al1.54 Cr0.42 Fe0.04 ) O4 ; the spinel in this 6 dunite is more than 50% poorer in chromium than spinels from the other two peridotite 7 xenoliths in this study. Most of the iron is divalent and in the tetrahedral site (Fe3+/∑Fe = 8 0.16 ± 0.02). Olivine, Cpx, and Opx have Fe3+/∑Fe of 0.03 ± 0.08, 0.33 ± 0.55, and 0.10 9 ± 0.04, respectively. BSE images of this xenolith also show some alteration by the host 10 11 basaltic magma (Supp. Fig. 1c), similar to that seen in the harzburgite. Two pyroxene-rich xenoliths were analyzed for this study: a clinopyroxenite (SC- 12 1-66), and an olivine websterite (SC-1-70). The clinopyroxenite contains 95% Cpx, 5% 13 Opx, <1% Spl, and no Ol. The average spinel composition in this rock is 14 3+ VI IV (Mg 0.72 Fe2+ 0.28 ) (Al1.44 Cr0.50 Fe0.04 ) O4 . These spinels are similar in chromium content 15 to those in the dunite. Clinopyroxene and Opx in this sample have Fe3+/∑Fe of 0.12 ± 16 0.07 and 0.09 ± 0.03, respectively. The websterite is composed of 58% Opx, 32% Ol, 17 10% Cpx, and <1% Spl, and the spinels have a calculated average mineral formula of 18 3+ VI IV (Mg 0.78 Fe2+ 0.22 ) (Al1.91 Cr0.06 Fe0.03 ) O4 . Unlike all of the other xenoliths, the spinels in 19 this websterite have very little chromium. As in every peridotite xenolith, most of the 20 iron in the spinel structure from the clinopyroxenite and the websterite exists as 21 tetrahedrally coordinated Fe2+. Olivine, Cpx, and Opx in this websterite have Fe3+/∑Fe 22 of 0.08 ± 0.04, 0.29 ± 0.05, and 0.08 ± 0.04, respectively. BSE images of both 1 pyroxenite xenoliths show alteration along grain boundaries, which may be textural 2 indicators of metasomatism (Supp. Fig. 1d,e). 3 4 2. Methods 5 6 2.1. Electron probe analyses and mineral formulae recalculation 7 8 9 Major element analyses were carried out on thin sections of each xenolith with a JEOL Superprobe microanalyzer with five spectrometers at UCLA. We used an 10 accelerating voltage of 15 kv, a 15 nA current, 20 seconds counting time, and a focused 11 beam. Mineral Fe3+/∑Fe values were calculated from electron microprobe analyses 12 following Droop (1987). Uncertainties on the Fe3+/∑Fe ratios were calculated using 13 Monte Carlo techniques and the 1 sigma uncertainties associated with the oxides in each 14 average mineral composition -- note that variations in SiO2 and FeO* have the largest 15 effect on calculated Fe3+ values (Canil and O’Neill, 1996). The reported Fe3+/∑Fe 16 errors (Table 1) are 2 standard deviations and are based on 100000 iterations per 17 calculation. 18 19 2.2. Column Chemistry 20 21 We used pre-filled Poly-Prep® columns measuring 4 cm x 0.8 cm with 10 ml 22 reservoirs for the two purification steps. These columns contain 0.3 ml (wet) of Bio- 23 Rad™ AG 1-X8 analytical grade resin in 200 to 400 mesh chloride form. Columns were 1 washed initially 3 times with 10 ml of 7N HCl alternating with ~18 MΩ cm2/cm water 2 and conditioned with 0.5N HCl and 7N HCl. A typical load on the column consists of 3 between 10 and 50 g of Fe in 300 l of 7N HCl. Matrix elements, including Ca, Mg, 4 Al, and Cr, are eluted by passing 9 ml of 7N HCl through the column, leaving Fe adhered 5 to the resin. Iron is then recovered by passing 1.5 ml of 0.5N HCl through the column. 6 The pure iron eluent is subsequently evaporated on a 120 °C hotplate until just dry, then 7 immediately picked up in 1 ml 2% HNO3 for mass spectrometry. Columns are used only 8 once, then discarded. 9 Elution curves were determined from synthetic dissolved rock solutions 10 containing various concentrations of Al, Mg, Ca, Fe, Cr, Mn, Mg and Ti. These rock and 11 mineral “analogues” were made by mixing varying amounts of Spex Certi-Prep standard 12 solutions to simulate the differing proportions of each element in typical Ol, Cpx, Opx, 13 and Spl from San Carlos xenoliths as determined by EMPA analyses of the xenoliths 14 from this study. The most reliable indicator of complete recovery of Fe in the presence 15 of matrix elements on the columns was the absence of measurable shifts in 56Fe/54Fe and 16 57 17 during this study. In other words, every time we carried out the column chemistry 18 procedure, one or more of the columns was loaded with a mineral analogue solution 19 instead of an actual dissolved mineral in order to test the column chemistry procedure for 20 the particular composition(s) of the mineral being purified. These “column tests” allow 21 for a high degree of confidence with regard to the isotope-ratio fidelity of our column 22 chemistry procedure. Fe/54Fe following Fe recovery. These “zero enrichments” were checked routinely 1 Tests show that even extremely small amounts of Cr will cause interferences and 2 matrix effects, resulting in erroneously low 57Fe and 56Fe values. This problem was 3 solved by repeating the column chemistry procedure with the Fe + Cr eluents. Repeating 4 the Fe column chemistry procedure for a single sample results in complete recovery of Fe 5 with no measurable shifts in 56Fe/54Fe and 57Fe/54Fe and elimination of Cr. Therefore, we 6 repeated the column separation procedure for spinel and whole rock samples in this 7 study. 8 9 References 10 11 12 13 Canil D. and O'Neill, H. S. C. (1996) Distribution of ferric iron in some upper-mantle assemblages. Journal of Petrology 37(3), 609-635. Droop G. T. R. (1987) A general equation for estimating Fe3+ concentrations in 14 ferromagnesian silicates and oxides from microprobe analyses, using 15 stoichiometric criteria. Mineralogical magazine 51(361), 431-435. 16 Frey F. A. and Prinz M. (1978) Ultramafic inclusions from San Carlos, Arizona: 17 Petrologic and geochemical data bearing on their petrogenesis. Earth and 18 Planetary Science Letters 38,129-176. 19 Galer S. J. G. and O’Nions R. K. (1988) Chemical and isotopic studies of ultramafic 20 inclusions from the San Carlos Volcanic Field, Arizona: A bearing on their 21 petrogenesis. Journal of Petrology 30, 1033-1064. 22 23 Menzies M. (2003) Mantle ultramafic xenoliths in alkaline magmas: evidence for mantle heterogeneity modified by magmatic activity. In C. J. Hawkesworth and M. J. 1 Norry (eds.) Continental Basalts and Mantle Xenoliths, Shiva, Cheshire, pp. 92- 2 110. 3 Wan Z., Coogan, L. A., and Canil, D. (2008) Experimental calibration of aluminum 4 partitioning between olivine and spinel as a geothermometer. American 5 Mineralogist 93(7), 1142-1147. 6 Wilshire H. G. and Shervais J. W. (1975) Al-augite and Cr-diopside ultramafic xenoliths 7 in basaltic rocks from western United States. Physics and Chemistry of the Earth 8 9, 257-272. 9 10 11 12 13 Wilshire H. G. and Jackson E. D. (1975) Problems in determining mantle geotherms from pyroxene compositions of ultramafic rocks. Journal of Geology 83, 313-329.